Earth:Water treatment

Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.
Types
Drinking water treatment
Water contamination is primarily caused by the discharge of untreated wastewater from enterprises. The effluent from various enterprises, which contains varying levels of contaminants, is dumped into rivers or other water resources. The wastewater may have a high proportion of organic and inorganic contaminants at the initial discharge. Industries generate wastewater as a result of fabrication processes, processes dealing with paper and pulp, textiles, chemicals, and from various streams such as cooling towers, boilers, and production lines.[1]
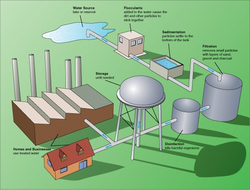
Treatment for drinking water production involves the removal of contaminants and/or inactivation of any potentially harmful microbes from raw water to produce water that is pure enough for human consumption without any short term or long term risk of any adverse health effect. In general terms, the greatest microbial risks are associated with ingestion of water that is contaminated with human or animal (including bird) faeces. Faeces can be a source of pathogenic bacteria, viruses, protozoa and helminths. The removal or destruction of microbial pathogens is essential, and commonly involves the use of reactive chemical agents such as suspended solids, to remove bacteria, algae, viruses, fungi, and minerals including iron and manganese. Research including Professor Linda Lawton's group at Robert Gordon University, Aberdeen is working to improve detection of cyanobacteria.[2] These substances continue to cause great harm to several less developed countries who do not have access to effective water purification systems.[original research?]
Measures taken to ensure water quality not only relate to the treatment of the water, but to its conveyance and distribution after treatment. It is therefore common practice to keep residual disinfectants in the treated water to kill bacteriological contamination during distribution and to keep the pipes clean.[3]
Water supplied to domestic properties such as for tap water or other uses, may be further treated before use, often using an in-line treatment process. Such treatments can include water softening or ion exchange. [citation needed]
Wastewater treatment
Industrial water treatment

Processes

For the elimination of hazardous chemicals from the water, many treatment procedures have been applied.[4]
The processes involved in removing the contaminants include physical processes such as settling and filtration, chemical processes such as disinfection and coagulation, and biological processes such as slow sand filtration.
A combination selected from the following processes (depending on the season and contaminants and chemicals present in the raw water) is used for municipal drinking water treatment worldwide.
Chemical

Different chemical procedures for the conversion into final products or the removal of pollutants are used for the safe disposal of contaminants.[5]
- Pre-chlorination for algae control and arresting biological growth.
- Aeration along with pre-chlorination for removal of dissolved iron when present with relatively small amounts of manganese.
- Disinfection for killing bacteria, viruses and other pathogens, using chlorine, ozone and ultra-violet light.
Physical
Physical techniques of water/waste water treatment rely on physical phenomena to complete the removal process, rather than biological or chemical changes.[5]
Most common physical techniques are:
- Sedimentation is one of the most important main wastewater treatment procedures. Gravity settling is a method of separating particles from a fluid. The particle in suspension remains stable in quiescent conditions due to the decrease in water velocity throughout the water treatment process, following which the particles settle by gravitational force.[6][7] For solids separation that is the removal of suspended solids trapped in the floc.
- Filtration is the technique of removing pollutants based on their particle size. Pollutant removal from waste water permits water to be reused for a variety of purposes. The types of filters used in the procedure differ depending on the contaminants present in the water. Particle filtration and Membrane filtration are the two main forms of waste water filtration.[8]
- Dissolved air flotation (Degasification) is the process of removing dissolved gases from a solution . Henry's law states that the amount of dissolved gas in a liquid is proportionate to the partial pressure of the gas. Degasification is a low-cost method of removing carbon dioxide gas from waste water that raises the pH of the water by removing the gas.[5]
- Deaerator is used to reduce oxygen and nitrogen in boiler feed water applications.
Physico-chemical
Also referred to as "Conventional" Treatment
- Coagulation for flocculation. The addition of coagulants destabilizes colloidal suspensions by neutralizing their charges, resulting in the aggregation of smaller particles during the coagulation process.[9]
- Coagulant aids, also known as polyelectrolytes – to improve coagulation and for more robust floc formation.
- Polyelectrolytes or also known in the field as polymers, usually consist of either a positive or negative charge. The nature of the polyelectrolyte used is purely based on the source water characteristics of the treatment plant.
- These will usually be used in conjunction with a primary coagulant such as ferric chloride, ferric sulfate, or alum.
Chemical precipitation is a common process used to reduce heavy metals concentrations in wastewater. The dissolved metal ions are transformed to an insoluble phase by a chemical interaction with a precipitant agent such as lime. In industrial applications stronger alkalis may be used to effect complete precipitation. In drinking water treatment, the common-ion effect is often used to help reduce water hardness.[10]
Flotation uses bubble attachment to separate solids or dispersed liquids from a liquid phase.[11]
Membrane filtration
Membrane filtration has gotten a lot of attention for inorganic effluent treatment since it can remove not only suspended solids and organic components, but also inorganic pollutants such heavy metals. For heavy metal removal, several forms of membrane filtration, such as ultrafiltration, nanofiltration, and reverse osmosis, can be used depending on the particle size that can be maintained.[12][13]
Ion exchange
Ion exchange is a reversible ion exchange process in which an insoluble substance (resin) takes ions from an electrolytic solution and releases additional ions of the same charge in a chemically comparable amount without changing the resin's structure.[14][15]
Electrochemical treatment techniques
- Electrodialysis (ED)
- Membrane electrolysis (ME)
- Electrochemical precipitation (EP)
Adsorption
Adsorption is a mass transfer process in which a substance is transported from the liquid phase to the surface of a solid/liquid (adsorbent) and becomes physically and chemically bonded (adsorbate). Adsorption can be classified into two forms based on the type of attraction between the adsorbate and the adsorbent: physical and chemical adsorption, commonly known as physisorption and chemisorptions.[16][17]
Activated carbon
Activated carbons (ACs) or biological-activated carbon (BAC)[18] are effective adsorbents for a wide variety of contaminants. The adsorptive removal of color, aroma, taste, and other harmful organics and inorganics from drinking water and wastewater is one of their industrial applications.[19]
Both a high surface area and a large pore size can improve the efficiency of activated carbon. Activated carbon was utilized by a number of studies to remove heavy metals and other types of contaminants from wastewater. The cost of activated carbon is rising due to a shortage of commercial activated carbon (AC). Because of its high surface area, porosity, and flexibility, activated carbon has a lot of potential in wastewater treatment.[19]
Biological
This is the method by which dissolved and suspended organic chemical components are eliminated through biodegradation, in which an optimal amount of microorganism is given to re-enact the same natural self-purification process.[20] Through two distinct biological process, such as biological oxidation and biosynthesis, microorganisms can degrade organic materials in wastewater. Microorganisms involved in wastewater treatment produce end products such as minerals, carbon dioxide, and ammonia during the biological oxidation process. The minerals (products) remained in the wastewater and were discharged with the effluent. Microorganisms use organic materials in wastewater to generate new microbial cells with dense biomass that is eliminated by sedimentation throughout the biosynthesis process.[21]
Standards

Many developed countries specify standards to be applied in their own country. In Europe, this includes the European Drinking Water Directive[22] and in the United States the United States Environmental Protection Agency (EPA) establishes standards as required by the Safe Drinking Water Act. For countries without a legislative or administrative framework for such standards, the World Health Organization publishes guidelines on the standards that should be achieved.[23] China adopted its own drinking water standard GB3838-2002 (Type II) enacted by Ministry of Environmental Protection in 2002.[24]
Where drinking water quality standards do exist, most are expressed as guidelines or targets rather than requirements, and very few water standards have any legal basis or, are subject to enforcement.[25] Two exceptions are the European Drinking Water Directive and the Safe Drinking Water Act in the United States, which require legal compliance with specific standards.
Developing countries
Appropriate technology options in water treatment include both community-scale and household-scale point-of-use (POU) or self-supply designs.[26] Such designs may employ solar water disinfection methods, using solar irradiation to inactivate harmful waterborne microorganisms directly, mainly by the UV-A component of the solar spectrum, or indirectly through the presence of an oxide photocatalyst, typically supported TiO2 in its anatase or rutile phases.[27] Despite progress in SODIS technology, military surplus water treatment units like the ERDLator are still frequently used in developing countries. Newer military style Reverse Osmosis Water Purification Units (ROWPU) are portable, self-contained water treatment plants are becoming more available for public use.[28]
For waterborne disease reduction to last, water treatment programs that research and development groups start in developing countries must be sustainable by the citizens of those countries. This can ensure the efficiency of such programs after the departure of the research team, as monitoring is difficult because of the remoteness of many locations.
Energy Consumption: Water treatment plants can be significant consumers of energy. In California, more than 4% of the state's electricity consumption goes towards transporting moderate quality water over long distances, treating that water to a high standard.[29] In areas with high quality water sources which flow by gravity to the point of consumption, costs will be much lower. Much of the energy requirements are in pumping. Processes that avoid the need for pumping tend to have overall low energy demands. Those water treatment technologies that have very low energy requirements including trickling filters, slow sand filters, gravity aqueducts.
A 2021 study found that a large-scale water chlorination program in urban areas of Mexico massively reduced childhood diarrheal disease mortality rates.[30]
Materials
Stainless steels, such as Type 304L and 316L, are used extensively in the fabrication of water treatment plants due to their corrosion resistance to water and to the corrosivity of chlorination used for disinfection.[31][32]
See also
- Control of water pollution
- Clean Water Act
- Peak water (water supply & demand)
- Pulsed-power water treatment
- Solar water disinfection
- Raw water#Treatment
- Water purification
- Water quality
- Water softening
- Water supply
References
- ↑ Singh, N. B.; Nagpal, Garima; Agrawal, Sonal; Rachna (2018-08-01). "Water purification by using Adsorbents: A Review" (in en). Environmental Technology & Innovation 11: 187–240. doi:10.1016/j.eti.2018.05.006. ISSN 2352-1864. https://www.sciencedirect.com/science/article/pii/S2352186417302663.
- ↑ "Linda Lawton – 11th International Conference on Toxic Cyanobacteria" (in en-US). http://ictc11.org/speakers/linda-lawton/.
- ↑ "Chlorine". Drinking water inspectorate. https://www.dwi.gov.uk/consumers/learn-more-about-your-water/chlorine/.
- ↑ Jothirani, R.; Kumar, P. Senthil; Saravanan, A.; Narayan, Abishek S.; Dutta, Abhishek (2016-07-25). "Ultrasonic modified corn pith for the sequestration of dye from aqueous solution" (in en). Journal of Industrial and Engineering Chemistry 39: 162–175. doi:10.1016/j.jiec.2016.05.024. ISSN 1226-086X. https://www.sciencedirect.com/science/article/pii/S1226086X1630137X.
- ↑ 5.0 5.1 5.2 Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P. R.; Reshma, B. (2021-10-01). "Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development" (in en). Chemosphere 280: 130595. doi:10.1016/j.chemosphere.2021.130595. ISSN 0045-6535. PMID 33940449. Bibcode: 2021Chmsp.280m0595S. https://www.sciencedirect.com/science/article/pii/S0045653521010663.
- ↑ Gottfried, A.; Shepard, A. D.; Hardiman, K.; Walsh, M. E. (2008-11-01). "Impact of recycling filter backwash water on organic removal in coagulation–sedimentation processes" (in en). Water Research 42 (18): 4683–4691. doi:10.1016/j.watres.2008.08.011. ISSN 0043-1354. PMID 18789473. https://www.sciencedirect.com/science/article/pii/S0043135408003291.
- ↑ Samal, Sneha (2020-04-15). "Effect of shape and size of filler particle on the aggregation and sedimentation behavior of the polymer composite" (in en). Powder Technology 366: 43–51. doi:10.1016/j.powtec.2020.02.054. ISSN 0032-5910. https://www.sciencedirect.com/science/article/pii/S0032591020301613.
- ↑ Ahmad, Arslan; Rutten, Sam; de Waal, Luuk; Vollaard, Peter; van Genuchten, Case; Bruning, Harry; Cornelissen, Emile; van der Wal, Albert (2020-06-15). "Mechanisms of arsenate removal and membrane fouling in ferric based coprecipitation–low pressure membrane filtration systems" (in en). Separation and Purification Technology 241: 116644. doi:10.1016/j.seppur.2020.116644. ISSN 1383-5866.
- ↑ Nyström, Fredrik; Nordqvist, Kerstin; Herrmann, Inga; Hedström, Annelie; Viklander, Maria (2020-09-01). "Removal of metals and hydrocarbons from stormwater using coagulation and flocculation" (in en). Water Research 182: 115919. doi:10.1016/j.watres.2020.115919. ISSN 0043-1354. PMID 32622122.
- ↑ Wang, Lawrence K.; Vaccari, David A.; Li, Yan; Shammas, Nazih K. (2005), "Chemical Precipitation", Physicochemical Treatment Processes (Totowa, NJ: Humana Press): pp. 141–197, doi:10.1385/1-59259-820-x:141, ISBN 978-1-58829-165-3, http://dx.doi.org/10.1385/1-59259-820-x:141, retrieved 2021-11-12
- ↑ Wang, Lawrence K.; Fahey, Edward M.; Wu, Zucheng (2005), Wang, Lawrence K.; Hung, Yung-Tse; Shammas, Nazih K., eds., "Dissolved Air Flotation" (in en), Physicochemical Treatment Processes (Totowa, NJ: Humana Press): pp. 431–500, doi:10.1385/1-59259-820-x:431, ISBN 978-1-58829-165-3, http://link.springer.com/10.1385/1-59259-820-x:431, retrieved 2021-11-12
- ↑ Chadha, Utkarsh; Selvaraj, Senthil Kumaran; Vishak Thanu, S.; Cholapadath, Vishnu; Abraham, Ashesh Mathew; Zaiyan, Mohammed; Manikandan, M; Paramasivam, Velmurugan (6 January 2022). "A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater". Materials Research Express 9 (1): 012003. doi:10.1088/2053-1591/ac48b8. Bibcode: 2022MRE.....9a2003C.
- ↑ 13.0 13.1 Kurniawan, Tonni Agustiono; Chan, Gilbert Y. S.; Lo, Wai-Hung; Babel, Sandhya (2006-05-01). "Physico–chemical treatment techniques for wastewater laden with heavy metals" (in en). Chemical Engineering Journal 118 (1): 83–98. doi:10.1016/j.cej.2006.01.015. ISSN 1385-8947. https://www.sciencedirect.com/science/article/pii/S1385894706000362.
- ↑ Vigneswaran, Saravanamuthu; Ngo, Huu Hao; Chaudhary, Durgananda Singh; Hung, Yung-Tse (2005), "Physicochemical Treatment Processes for Water Reuse", Physicochemical Treatment Processes (Totowa, NJ: Humana Press): pp. 635–676, doi:10.1385/1-59259-820-x:635, ISBN 978-1-58829-165-3, http://dx.doi.org/10.1385/1-59259-820-x:635, retrieved 2021-11-12
- ↑ Rengaraj, S; Yeon, Kyeong-Ho; Moon, Seung-Hyeon (October 2001). "Removal of chromium from water and wastewater by ion exchange resins". Journal of Hazardous Materials 87 (1–3): 273–287. doi:10.1016/s0304-3894(01)00291-6. ISSN 0304-3894. PMID 11566415. http://dx.doi.org/10.1016/s0304-3894(01)00291-6.
- ↑ Singh, N. B.; Nagpal, Garima; Agrawal, Sonal; Rachna (2018-08-01). "Water purification by using Adsorbents: A Review" (in en). Environmental Technology & Innovation 11: 187–240. doi:10.1016/j.eti.2018.05.006. ISSN 2352-1864. https://www.sciencedirect.com/science/article/pii/S2352186417302663.
- ↑ BABEL, Sandhya; KURNIAWAN, Tonni Agustiono (2003). "A Research Study on Cr(VI) Removal from Contaminated Wastewater Using Natural Zeolite". Journal of Ion Exchange 14 (Supplement): 289–292. doi:10.5182/jaie.14.supplement_289. ISSN 1884-3360. Bibcode: 2003JIEx...14S.289B.
- ↑ Sirotkin, A.; Koshkina, L. Yu.; Ippolitov, K. G. (2001). "The BAC-process for treatment of waste water Containing non-ionogenic synthetic surfactants". Water Research 35 (13): 3265–3271. doi:10.1016/s0043-1354(01)00029-x. PMID 11487125.
- ↑ 19.0 19.1 Mezohegyi, Gergo; van der Zee, Frank P.; Font, Josep; Fortuny, Agustí; Fabregat, Azael (2012-07-15). "Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon" (in en). Journal of Environmental Management 102: 148–164. doi:10.1016/j.jenvman.2012.02.021. ISSN 0301-4797. PMID 22459012. https://www.sciencedirect.com/science/article/pii/S0301479712000904.
- ↑ GracePavithra, Kirubanandam; Jaikumar, V.; Kumar, P. Senthil; SundarRajan, PanneerSelvam (2019-08-10). "A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective" (in en). Journal of Cleaner Production 228: 580–593. doi:10.1016/j.jclepro.2019.04.117. ISSN 0959-6526. https://www.sciencedirect.com/science/article/pii/S0959652619311965.
- ↑ Gray, Nick (2017-01-31). Water Technology (3 ed.). London: CRC Press. doi:10.1201/9781315276106. ISBN 978-1-315-27610-6. https://www.taylorfrancis.com/books/mono/10.1201/9781315276106/water-technology-nick-gray.
- ↑ "Legislation: The Directive overview". Brussels: European Commission. 2019-12-31. https://ec.europa.eu/environment/water/water-drink/legislation_en.html.
- ↑ Guidelines for Drinking-water Quality, Fourth Edition; World Health Organization; 2011
- ↑ "Environmental quality standards for surface water". http://kjs.mep.gov.cn/hjbhbz/bzwb/shjbh/shjzlbz/200206/t20020601_66497.htm.
- ↑ What is the purpose of drinking water quality guidelines/regulations?. Canada: Safe Drinking Water Foundation. http://www.safewater.org/resources/fact-sheets.html. Pdf.
- ↑ "Household Water Treatment Guide". Centre for Affordable Water and Sanitation Technology, Canada. March 2008. http://www.cawst.org/en/resources/pubs.
- ↑ "Sand as a low-cost support for titanium dioxide photocatalysts". Materials Views. Wiley VCH. http://www.materialsviews.com/sand-as-a-low-cost-support-for-titanium-dioxide-photocatalysts/.
- ↑ Lindsten, Don C. (September 1984). "Technology transfer: Water purification, U.S. Army to the civilian community". The Journal of Technology Transfer 9 (1): 57–59. doi:10.1007/BF02189057.
- ↑ "Energy Costs of Water in California". http://large.stanford.edu/courses/2012/ph240/spearrin1/.
- ↑ Bhalotra, Sonia R.; Diaz-Cayeros, Alberto; Miller, Grant; Miranda, Alfonso; Venkataramani, Atheendar S. (2021). "Urban Water Disinfection and Mortality Decline in Lower-Income Countries" (in en). American Economic Journal: Economic Policy 13 (4): 490–520. doi:10.1257/pol.20180764. ISSN 1945-7731. https://www.aeaweb.org/articles?id=10.1257/pol.20180764.
- ↑ R.E. Avery, S. Lamb, C.A. Powell and A.H. Tuthill. "Stainless Steels for Potable Water Treatment Plants". https://nickelinstitute.org/en/library/technical-guides/stainless-steel-for-potable-water-treatment-plants-10087/.
- ↑ A.H. Tuthill and S. Lamb. "Guidelines for the use of Stainless Steel in Municipal Waste Water Treatment Plants". https://nickelinstitute.org/en/library/technical-guides/guidelines-for-the-use-of-stainless-steel-in-municipal-waste-water-treatment-plants-10076/.
External links
| Wikimedia Commons has media related to Water treatment. |
- International Water Association Professional / research organization
- NSF International – Independent non-profit standards organization
- WHO.int, WHO Guidelines
- Safe and Sustainable Water for Haiti web site hosted by Grand Valley State University
 |

