Engineering:Raman microscope


The Raman microscope is a laser-based microscopic device used to perform Raman spectroscopy.[1] The term MOLE (molecular optics laser examiner) is used to refer to the Raman-based microprobe.[1] The technique used is named after C. V. Raman, who discovered the scattering properties in liquids.[2]
Configuration
The Raman microscope begins with a standard optical microscope, and adds an excitation laser, laser rejection filters, a spectrometer or monochromator, and an optical sensitive detector such as a charge-coupled device (CCD), or photomultiplier tube, (PMT). Traditionally Raman microscopy was used to measure the Raman spectrum of a point on a sample, more recently the technique has been extended to implement Raman spectroscopy for direct chemical imaging over the whole field of view on a 3D sample.
Imaging modes
In direct imaging, the whole field of view is examined for scattering over a small range of wavenumbers (Raman shifts). For instance, a wavenumber characteristic for cholesterol could be used to record the distribution of cholesterol within a cell culture. The other approach is hyperspectral imaging or chemical imaging, in which thousands of Raman spectra are acquired from all over the field of view. The data can then be used to generate images showing the location and amount of different components. Taking the cell culture example, a hyperspectral image could show the distribution of cholesterol,[3] as well as proteins, nucleic acids, and fatty acids.[4][5][6] Sophisticated signal- and image-processing techniques can be used to ignore the presence of water, culture media, buffers, and other interference.
Resolution
Raman microscopy, and in particular confocal microscopy, can reach down to sub-micrometer lateral spatial resolution.[7] Because a Raman microscope is a diffraction-limited system, its spatial resolution depends on the wavelength of light and the numerical aperture of the focusing element. In confocal Raman microscopy, the diameter of the confocal aperture is an additional factor. As a rule of thumb, the lateral spatial resolution can reach approximately the laser wavelength when using air objective lenses, while oil or water immersion objectives can provide lateral resolutions of around half the laser wavelength. This means that when operated in the visible to near-infrared range, a Raman microscope can achieve lateral resolutions of approx. 1 µm down to 250 nm, while the depth resolution (if not limited by the optical penetration depth of the sample) can range from 1-6 µm with the smallest confocal pinhole aperture to tens of micrometers when operated without a confocal pinhole.[8][9][10] Since the objective lenses of microscopes focus the laser beam down to the micrometer range, the resulting photon flux is much higher than achieved in conventional Raman setups. This has the added effect of increased photobleaching of molecules emitting interfering fluorescence. However, the high photon flux can also cause sample degradation, and thus, for each type of sample, the laser wavelength and laser power have to be carefully selected.
Raman imaging
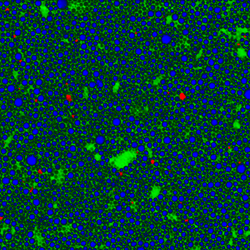
Another tool that is becoming more popular is global Raman imaging. This technique is being used for the characterization of large scale devices, mapping of different compounds and dynamics study. It has already been used for the characterization of graphene layers,[11] J-aggregated dyes inside carbon nanotubes and multiple other 2D materials such as MoS2[12] and WSe2. Since the excitation beam is dispersed over the whole field of view, those measurements can be done without damaging the sample. By using Raman microspectroscopy, in vivo time- and space-resolved Raman spectra of microscopic regions of samples can be measured. As a result, the fluorescence of water, media, and buffers can be removed. Consequently, it is suitable to examine proteins, cells and organelles.
Raman microscopy for biological and medical specimens generally uses near-infrared (NIR) lasers (785 nm diodes and 1064 nm Nd:YAG are especially common). This reduces the risk of damaging the specimen by applying higher energy wavelengths. However, the intensity of NIR Raman scattering is low (owing to the ω4 dependence of Raman scattering intensity), and most detectors require very long collection times. Recently, more sensitive detectors have become available, making the technique better suited to general use. Raman microscopy of inorganic specimens, such as rocks, ceramics and polymers,[13] can use a broader range of excitation wavelengths.
A related technique, tip-enhanced Raman spectroscopy, can produce high-resolution hyperspectral images of single molecules[14] and DNA.[15]
Correlative Raman imaging

Confocal Raman microscopy can be combined with numerous other microscopy techniques. By using different methods and correlating the data, the user attains a more comprehensive understanding of the sample. Common examples of correlative microscopy techniques are Raman-AFM,[16][13] Raman-SNOM,[17] and Raman-SEM.[18]
Correlative SEM-Raman imaging is the integration of a confocal Raman microscope into an SEM chamber which allows correlative imaging of several techniques, such as SE, BSE, EDX, EBSD, EBIC, CL, AFM.[19] The sample is placed in the vacuum chamber of the electron microscope. Both analysis methods are then performed automatically at the same sample location. The obtained SEM and Raman images can then be superimposed.[20][21] Moreover, adding a focused ion beam (FIB) on the chamber allows removal of the material and therefore 3D imaging of the sample. Low-vacuum mode allows analysis on biological and non-conductive samples.
Biological Applications
By using Raman microspectroscopy, in vivo time- and space-resolved Raman spectra of microscopic regions of samples can be measured. Sampling is non-destructive and water, media, and buffers typically do not interfere with the analysis. Consequently, in vivo time- and space-resolved Raman spectroscopy is suitable to examine proteins, cells and organs. In the field of microbiology, confocal Raman microspectroscopy has been used to map intracellular distributions of macromolecules, such as proteins, polysaccharides, and nucleic acids and polymeric inclusions, such as poly-β-hydroxybutyric acid and polyphosphates in bacteria and sterols in microalgae. Combining stable isotopic probing (SIP) experiments with confocal Raman microspectroscopy has permitted determination of assimilation rates of 13C and 15N-substrates as well as D2O by individual bacterial cells.[22]
See also
- Raman scattering
- Coherent Raman Scattering Microscopy
- Scanning electron microscope
- Tip-enhanced Raman spectroscopy
References
- ↑ 1.0 1.1 Microscopical techniques in the use of the molecular optics laser examiner Raman microprobe, by M. E. Andersen, R. Z. Muggli, Analytical Chemistry, 1981, 53 (12), pp 1772–1777 [1]
- ↑ Krishnan, K. S.; Raman, C. V. (1928). "A New Type of Secondary Radiation". Nature 121 (3048): 501–502. doi:10.1038/121501c0. ISSN 1476-4687. Bibcode: 1928Natur.121..501R.
- ↑ Matthäus, Christian; Krafft, Christoph; Dietzek, Benjamin; Brehm, Bernhard R.; Lorkowski, Stefan; Popp, Jürgen (2012-10-16). "Noninvasive Imaging of Intracellular Lipid Metabolism in Macrophages by Raman Microscopy in Combination with Stable Isotopic Labeling". Analytical Chemistry 84 (20): 8549–8556. doi:10.1021/ac3012347. ISSN 0003-2700. PMID 22954250.
- ↑ Baranska, Malgorzata; Chlopicki, Stefan; Fedorowicz, Andrzej; Kachamakova-Trojanowska, Neli; Kaczor, Agnieszka; Majzner, Katarzyna (2012-12-10). "3D confocal Raman imaging of endothelial cells and vascular wall: perspectives in analytical spectroscopy of biomedical research". Analyst 138 (2): 603–610. doi:10.1039/C2AN36222H. ISSN 1364-5528. PMID 23172339.
- ↑ Rygula, A.; Majzner, K.; Marzec, K. M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. (2013-08-01). "Raman spectroscopy of proteins: a review". Journal of Raman Spectroscopy 44 (8): 1061–1076. doi:10.1002/jrs.4335. ISSN 1097-4555. Bibcode: 2013JRSp...44.1061R.
- ↑ Czamara, K.; Majzner, K.; Pacia, M. Z.; Kochan, K.; Kaczor, A.; Baranska, M. (2015-01-01). "Raman spectroscopy of lipids: a review". Journal of Raman Spectroscopy 46 (1): 4–20. doi:10.1002/jrs.4607. ISSN 1097-4555. Bibcode: 2015JRSp...46....4C.
- ↑ Toporski, Jan; Dieing, Thomas; Hollricher, Olaf, eds (2018). Confocal Raman Microscopy. Springer Series in Surface Sciences. 66. doi:10.1007/978-3-319-75380-5. ISBN 978-3-319-75378-2. https://cds.cern.ch/record/1339422.
- ↑ Neil J. Everall (2009). "Confocal Raman Microscopy: Performance, Pitfalls, and Best Practice". Applied Spectroscopy 63 (9): 245A–262A. doi:10.1366/000370209789379196. ISSN 1943-3530. PMID 19796478. Bibcode: 2009ApSpe..63..245E.
- ↑ Supporting Information of T. Schmid; N. Schäfer; S. Levcenko; T. Rissom; D. Abou-Ras (2015). "Orientation-distribution mapping of polycrystalline materials by Raman microspectroscopy". Scientific Reports 5: 18410. doi:10.1038/srep18410. ISSN 2045-2322. PMID 26673970. Bibcode: 2015NatSR...518410S.
- ↑ Lothar Opilik; Thomas Schmid; Renato Zenobi (2013). "Modern Raman Imaging: Vibrational Spectroscopy on the Micrometer and Nanometer Scales". Annual Review of Analytical Chemistry 6: 379–398. doi:10.1146/annurev-anchem-062012-092646. ISSN 1936-1335. PMID 23772660. Bibcode: 2013ARAC....6..379O.
- ↑ Shen, Zexiang; Yu, Ting; Wang, Yingying; Ni, Zhenhua (2008-10-01). "Raman spectroscopy and imaging of graphene". Nano Research 1 (4): 273–291. doi:10.1007/s12274-008-8036-1. ISSN 1998-0000.
- ↑ Li, Hai; Lu, Gang; Yin, Zongyou; He, Qiyuan; Li, Hong; Zhang, Qing; Zhang, Hua (2012-03-12). "Optical Identification of Single- and Few-Layer MoS2 Sheets". Small 8 (5): 682–686. doi:10.1002/smll.201101958. ISSN 1613-6829. PMID 22223545.
- ↑ 13.0 13.1 Schmidt, U.; Hild, S.; Ibach, W.; Hollricher, O. (2005-12-01). "Characterization of Thin Polymer Films on the Nanometer Scale with Confocal Raman AFM". Macromolecular Symposia 230 (1): 133–143. doi:10.1002/masy.200551152. ISSN 1521-3900.
- ↑ Apkarian, V. Ara; Nicholas Tallarida; Crampton, Kevin T.; Lee, Joonhee (April 2019). "Visualizing vibrational normal modes of a single molecule with atomically confined light". Nature 568 (7750): 78–82. doi:10.1038/s41586-019-1059-9. ISSN 1476-4687. PMID 30944493. Bibcode: 2019Natur.568...78L.
- ↑ He, Zhe; Han, Zehua; Kizer, Megan; Linhardt, Robert J.; Wang, Xing; Sinyukov, Alexander M.; Wang, Jizhou; Deckert, Volker et al. (2019-01-16). "Tip-Enhanced Raman Imaging of Single-Stranded DNA with Single Base Resolution". Journal of the American Chemical Society 141 (2): 753–757. doi:10.1021/jacs.8b11506. ISSN 0002-7863. PMID 30586988.
- ↑ Pilarczyk, Marta; Rygula, Anna; Kaczor, Agnieszka; Mateuszuk, Lukasz; Maślak, Edyta; Chlopicki, Stefan; Baranska, Malgorzata (2014-11-01). "A novel approach to investigate vascular wall in 3D: Combined Raman spectroscopy and atomic force microscopy for aorta en face imaging". Vibrational Spectroscopy 75: 39–44. doi:10.1016/j.vibspec.2014.09.004. ISSN 0924-2031.
- ↑ Stark, Robert W.; Hillenbrand, Rainer; Ziegler, Alexander; Bauer, Michael; Huber, Andreas J.; Gigler, Alexander M. (2009-12-07). "Nanoscale residual stress-field mapping around nanoindents in SiC by IR s-SNOM and confocal Raman microscopy". Optics Express 17 (25): 22351–22357. doi:10.1364/OE.17.022351. ISSN 1094-4087. PMID 20052158. Bibcode: 2009OExpr..1722351G.
- ↑ Cardell, Carolina; Guerra, Isabel (2016-03-01). "An overview of emerging hyphenated SEM-EDX and Raman spectroscopy systems: Applications in life, environmental and materials sciences". TrAC Trends in Analytical Chemistry 77: 156–166. doi:10.1016/j.trac.2015.12.001. ISSN 0165-9936.
- ↑ Jiruše, Jaroslav; Haničinec, Martin; Havelka, Miloslav; Hollricher, Olaf; Ibach, Wolfram; Spizig, Peter (2014). "Integrating focused ion beam–scanning electron microscope with confocal Raman microscope into a single instrument". Journal of Vacuum Science & Technology B, Nanotechnology and Microelectronics: Materials, Processing, Measurement, and Phenomena 32 (6): 06FC03. doi:10.1116/1.4897502.
- ↑ Hollricher, Olaf; Schmidt, Ute; Breuninger, Sonja (November 2014). "RISE Microscopy: Correlative Raman-SEM Imaging". Microscopy Today 22 (6): 36–39. doi:10.1017/s1551929514001175. ISSN 1551-9295.
- ↑ Wille, G.; Lerouge, C.; Schmidt, U. (2018-06-01). "A multimodal microcharacterisation of trace-element zonation and crystallographic orientation in natural cassiterite by combining cathodoluminescence, EBSD, EPMA and contribution of confocal Raman-in-SEM imaging". Journal of Microscopy 270 (3): 309–317. doi:10.1111/jmi.12684. ISSN 1365-2818. PMID 29336485.
- ↑ Madigan, M.T., Bender, K.S., Buckley, D.H., Sattley, W.M. and Stahl, D.A. (2018) Brock Biology of Microorganisms, Pearson Publ., NY, NY, 1022 pp.
 |

