Biology:Bethesda system
The Bethesda system (TBS), officially called The Bethesda System for Reporting Cervical Cytology, is a system for reporting cervical or vaginal cytologic diagnoses,[1] used for reporting Pap smear results. It was introduced in 1988[2] and revised in 1991,[3] 2001,[1][4][5] and 2014.[6] The name comes from the location (Bethesda, Maryland) of the conference, sponsored by the National Institutes of Health, that established the system.
Since 2010, there is also a Bethesda system used for cytopathology of thyroid nodules, which is called The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC or BSRTC). Like TBS, it was the result of a conference sponsored by the NIH and is published in book editions (currently by Springer). Mentions of "the Bethesda system" without further specification usually refer to the cervical system, unless the thyroid context of a discussion is implicit.
Cervix
Abnormal results include:[citation needed]
- Atypical squamous cells
- Atypical squamous cells of undetermined significance (ASC-US)
- Atypical squamous cells – cannot exclude HSIL (ASC-H)
- Low-grade squamous intraepithelial lesion (LGSIL or LSIL)
- High grade squamous intraepithelial lesion (HGSIL or HSIL)
- Squamous cell carcinoma
- Atypical Glandular Cells not otherwise specified (AGC-NOS)
- Atypical Glandular Cells, suspicious for AIS or cancer (AGC-neoplastic)
- Adenocarcinoma in situ (AIS)
The results are calculated differently following a Pap smear of the cervix.[citation needed]
Squamous cell abnormalities
LSIL: low-grade squamous intraepithelial lesion
A low-grade squamous intraepithelial lesion (LSIL or LGSIL) indicates possible cervical dysplasia. LSIL usually indicates mild dysplasia (CIN 1), more than likely caused by a human papillomavirus infection. It is usually diagnosed following a Pap smear.[citation needed]
CIN 1 is the most common and most benign form of cervical intraepithelial neoplasia and usually resolves spontaneously within two years. Because of this, LSIL results can be managed with a simple "watch and wait" philosophy. However, because there is a 12–16% chance of progression to more severe dysplasia, the physician may want to follow the results more aggressively by performing a colposcopy with biopsy.[7] If the dysplasia progresses, treatment may be necessary. Treatment involves removal of the affected tissue, which can be accomplished by LEEP, cryosurgery, cone biopsy, or laser ablation.[citation needed]
HSIL: high-grade squamous intraepithelial lesion
High-grade squamous intraepithelial lesion (HSIL or HGSIL) indicates moderate or severe cervical intraepithelial neoplasia or carcinoma in situ. It is usually diagnosed following a Pap test. In some cases these lesions can lead to invasive cervical cancer, if not followed appropriately.[citation needed]
HSIL does not mean that cancer is present. Of all women with HSIL results, 2%[8] or less[9] have invasive cervical cancer at that time, however about 20% would progress to having invasive cervical cancer without treatment.[10] To combat this progression, HSIL is usually followed by an immediate colposcopy with biopsy to sample or remove the dysplastic tissue. This tissue is sent for pathology testing to assign a histologic classification that is more definitive than a Pap smear result (which is a cytologic finding). HSIL generally corresponds to the histological classification of CIN 2 or 3.[citation needed]
HSIL treatment involves the removal or destruction of the affected cells, usually by LEEP. Other methods include cryotherapy, cautery, or laser ablation, but none are performed on pregnant women for fear of disrupting the pregnancy.[11] Any of these procedures is 85% likely to cure the problem.
Glandular cell abnormalities
Adenocarcinoma
Adenocarcinoma can arise from the endocervix, endometrium and extrauterine sites.[citation needed]
AGC
AGC, formerly AGUS, is a term for atypical glandular cells of undetermined significance.[12] Renamed AGC to avoid confusion with ASCUS.[1]
The management of AGC is colposcopy with or without an endometrial biopsy.[citation needed]
Thyroid nodules
The Bethesda System for Reporting Thyroid Cytopathology is the system used to report whether the thyroid cytological specimen is benign or malignant on fine-needle aspiration cytology (FNAC). It can be divided into six categories:
| Category | Description | Risk of malignancy[13] | Recommendation[13] |
|---|---|---|---|
| I | Non diagnostic/unsatisfactory | - | Repeating FNAC with ultrasound-guidance in more than 3 months |
| II | Benign (colloid and follicular cells) | 0 - 3% | Clinical follow-up |
| III | Atypia of undetermined significance/follicular lesion of undetermined significance (follicular or lymphoid cells with atypical features) | 5 - 15% | Repeating FNAC |
| IV | Follicular nodule/suspicious follicular nodule (cell crowding, micro follicles, dispersed isolated cells, scant colloid) | 15 - 30% | Surgical lobectomy |
| V | Suspicious for malignancy | 60 - 75% | Surgical lobectomy or near-total thyroidectomy |
| VI | Malignant | 97 - 99% | Near-total thyroidectomy |
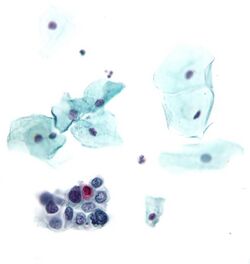
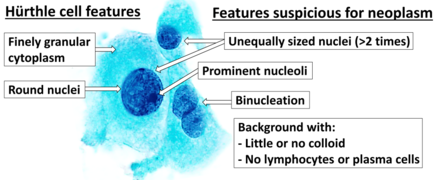
Cytopathology suspicious for Hürthle cell neoplasm (Bethesda category IV, rather than Hürthle cell hyperplasia), Pap stain.[14]
Repeated FNAC is recommended for Category I, followed by clinical follow-up in Category II, repeat FNAC for Category III, and lobectomy for Category IV, near total-thyroidectomy/lobectomy for Category V, and near total thyroidectomy for Category VI.[15] The risk of malignancy in a malignant FNAC report is 93.7% while for a suspicious FNAC report, it is 18.9%.[16]
See also
- American Society for Clinical Pathology
References
- ↑ 1.0 1.1 1.2 "The 2001 Bethesda System terminology". Am Fam Physician 68 (10): 1992–8. November 2003. PMID 14655809. http://www.aafp.org/afp/20031115/1992.html. Retrieved 2009-01-03.
- ↑ Soloman, Diane (1989). "The 1988 Bethesda System for reporting cerval/vaginal cytologic diagnoses: developed and approved at the National Cancer Institute workshop in Bethesda, MD, December 12–13, 1988". Diagn. Cytopathol. 5 (3): 331–4. doi:10.1002/dc.2840050318. PMID 2791840.
- ↑ Broder S (1992). "The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses—Report of the 1991 Bethesda Workshop". JAMA 267 (14): 1892. doi:10.1001/jama.1992.03480140014005.
- ↑ Nayar R, Solomon D. Second edition of 'The Bethesda System for reporting cervical cytology' – Atlas, website, and Bethesda interobserver reproducibility project. CytoJournal [serial online] 2004 [cited 2011 Apr 17];1:4. Available from: http://www.cytojournal.com/text.asp?2004/1/1/4/41272
- ↑ "The 2001 Bethesda System: terminology for reporting results of cervical cytology". JAMA 287 (16): 2114–9. April 2002. doi:10.1001/jama.287.16.2114. PMID 11966386. http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=11966386.
- ↑ Nayar R, Wilbur D. The Bethesda System for Reporting Cervical Cytology, Definitions, Criteria, and Explanatory Notes. Springer; 2015.
- ↑ "2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests". Am J Obstet Gynecol 197 (4): 346–55. Oct 2007. doi:10.1016/j.ajog.2007.07.047. PMID 17904957.
- ↑ Massad LS; Collins YC; Meyer PM. Biopsy correlates of abnormal cervical cytology classified using the Bethesda system. Gynecologic Oncology. 2001 Sep;82(3):516-22.
- ↑ Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstetric Gynecology. 1998 Oct;92(4 Pt 2):727-35.
- ↑ McIndoe WA; McLean MR; Jones RW; Mullins PR. The invasive potential of carcinoma in situ of the cervix. Obstetric Gynecology. 1984 Oct;64(4):451-8.
- ↑ Wright TC Jr; Massad LS; Dunton CJ; Spitzer M; Wilkinson EJ; Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. American Journal of Obstetric Gynecology. 2007 Oct;197(4):346-55.
- ↑ AGUS at eMedicine Dictionary
- ↑ 13.0 13.1 Renuka, I. V.; Saila Bala, G.; Aparna, C.; Kumari, Ramana; Sumalatha, K. (December 2012). "The Bethesda System for Reporting Thyroid Cytopathology: Interpretation and Guidelines in Surgical Treatment". Indian Journal of Otolaryngology and Head & Neck Surgery 64 (4): 305–311. doi:10.1007/s12070-011-0289-4. PMID 24294568.
- ↑ Image by Mikael Häggström, MD. References for findings:
- Ayana Suzuki, C.T., Andrey Bychkov, M.D., Ph.D.. "Hürthle cell neoplasm". https://www.pathologyoutlines.com/topic/thyroidhurthlecellneoplasm.html. Last author update: 7 May 2020. Last staff update: 12 May 2022
- Shawky M, Sakr M (2016). "Hurthle Cell Lesion: Controversies, Challenges, and Debates.". Indian J Surg 78 (1): 41–8. doi:10.1007/s12262-015-1381-x. PMID 27186039. - ↑ Renuka, I.V; Saila Bala, G; Aparna, C; Kumari, R; Sumalatha, K (December 2012). "The Bethesda System for Reporting Thyroid Cytopathology: Interpretation and Guidelines in Surgical Treatment". Indian J Otolaryngol Head Neck Surg 64 (4): 305–311. doi:10.1007/s12070-011-0289-4. PMID 24294568.
- ↑ Tee, Yoon Y; Lowe, Adrain J; Brand, Caroline A (November 2007). "Fine-Needle Aspiration May Miss a Third of All Malignancy in Palpable Thyroid Nodules". Annals of Surgery 246 (5): 714–720. doi:10.1097/SLA.0b013e3180f61adc. PMID 17968160. "our study showed that the risk of malignancy of malignant FNA and suspicious FNA diagnosis is around 93.7% and 18.9%, respectively.".
External links
- ASCP: The Bethesda System Website Atlas
- Bethesda 2001 Workshop
- Bongiovanni, Massimo; Spitale, Alessandra; Faquin, William C.; Mazzucchelli, Luca; Baloch, Zubair W. (2012). "The Bethesda System for Reporting Thyroid Cytopathology: A Meta-Analysis". Acta Cytologica 56 (4): 333–339. doi:10.1159/000339959. PMID 22846422. http://www.thyroid.org/professionals/ata-publications/clinical-thyroidology/january-2013-volume-25-issue-1/clin-thyroidol-20132516-17/. Retrieved 24 November 2022.
 |