Medicine:Convection enhanced delivery
Convection-enhanced delivery (CED) is method of drug delivery in which drug is delivered into using bulk flow rather than conventional diffusion into the brain. This is done by utilizing catheters inserted into the target region of the brain and utilizing pressure to deliver the therapeutic to a target region. CED has been used to delivery drugs to the central nervous system (CNS) for diseases such as cancer, epilepsy, and Parkinson’s disease.[1][2][3] CED has been used to deliver drugs to the CNS for its ability to bypass the blood–brain barrier (BBB) and target specific regions for targeted treatment, but current techniques using CED have failed to progress past clinical trials due to a variety of physical limitations associated with CED itself.
Background
The blood brain barrier (BBB) has historically proved to be a very difficult obstacle to overcome when aiming to deliver a drug to the brain. In order to overcome the difficulties in delivering therapeutic levels of drug past the BBB, drugs had to either be lipophilic molecules with a molecular weight below 600 Da or be transported across the BBB using some sort of cellular transport system.[4] In the 1990s, a research group led by Edward Oldfield at the National Institutes of Health proposed utilizing CED to deliver drugs and molecules too big to bypass the BBB to the brain.[5] CED is also useful to delivery drugs that have poor diffusive properties and allows for targeted placement of the catheter used to deliver the drugs. A vast majority of current clinical studies using CED are currently using CED as a method to treat brain tumors that are inoperable or have shown little response to conventional therapies.
Mechanism of action
CED is a method of drug delivery in which a pressure gradient is created at the tip of a catheter to use bulk flow rather than diffusion to delivery drugs into the brain. Diffusion has been limited by the diffusivity of the tissue, and can be expressed using Fick’s law, , where J is diffusion, D is the diffusivity of the targeted tissue, and is the concentration gradient of the drug. Diffusion is can only be modified by the concentration gradient of a drug, meaning that in order to deliver drug to large parts of a tissue, high concentrations of a drug are needed in order to promote diffusion, which can result in toxicity. In comparison, bulk flow is limited only by Darcy’s law, defined as , where v is velocity, K is the hydraulic conductivity of the molecule, and is the pressure gradient. Using bulk flow to deliver a drug can mean a drug can be delivered further into a target tissue with higher pressure, resulting in lower concentrations and less risk of drug toxicity.[6]
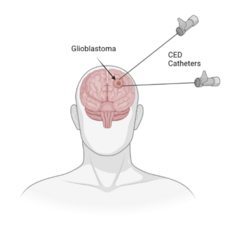
To perform a CED treatment, catheters are inserted through burr holes drilled into the skull. Treatments can use multiple catheters for a single delivery if that is required. The catheters are inserted into the interstitial space of the brain using image guidance. Once the catheters are placed at the desired site, the catheters are connection to an infusion pump which is used to create the pressure gradient for bulk flow. Infusion rates are typically set to 0.1-10 μL/min and the drug is delivered into the interstitial space, displacing any extracellular fluid.[5] CED can result in delivery of drug centimeters deep into the tissue from the delivery site, rather than the millimeters deep that would result from delivery of drugs via diffusion.[5]
Clinical trials evaluating CED
Current clinical trials exploring the use of CED to date have not resulted in any FDA approved treatments. These clinical trials have mostly been focused on using CED to treat glioblastoma and only two studies have been able to progress to stage 3 clinical trials. The first study began in 2004 and was comparing the efficacy of cintredekin besudotox delivered using CED and gliadel for the treatment of glioblastoma multiform.[7] Results from this study showed similar survival rates between the two groups, but patients who were given CED treatment had higher rates of pulmonary emboli. The second stage 3 clinical trial began in 2008 and was delivering trabedersen via CED to treat anaplastic astrocytoma glioblastoma.[8] This trial was terminated early due to the inability to recruit enough trial participants and efficacy of CED in this treatment was not established. These two studies have been the only major clinical trials which have compared the efficacy of CED treatment to current clinical standards for treatment.
While CED clinical trials have primarily explored treating brain tumors, other conditions involving the brain have also been investigated in clinical trials. To date there have been 2 registered clinical trials, both in stage 1, which aim to use CED to treat Parkinson’s disease. The first trial, which was registered in 2009, was withdrawn in 2017 for unknown reasons.[9] The other clinical trial, which reached completion in 2022, delivered an adenovirus (AAV2) encoding for a glial cell line derived neurotropic growth factor (GDNF) directly into the brain using CED.[10] GDNF is known to protect neurons which produce dopamine. Parkinson’s disease has been shown to decrease the amount of dopamine which can be produced in the brain, so researchers hope to be able to decrease the side effects of Parkinson’s disease by protecting neurons which produce dopamine. While results from this study have not been published as of April 2022, the pre-clinical research done in a Parkinson’s disease model rhesus monkey showed that CED treatment with AAV2-GDNF resulted in neurological improvement without significant side effects.[11]
Non-clinical research
Even though current clinical trials have not yet resulted in an FDA approved treatment, there is still plenty of research being done on delivering different types of therapeutics and treating different diseases being done. One of these areas of research is the visualization of the region of treatment. One research group was able visualize the regions of the brain that received drug from bulk flow mixing the desired drug with Gd-DTPA, a common MRI contrast agent.[12] This allowed researchers to immediately take an MRI post CED treatment to assess if the drug was reaching the targeted area. Research has also tagged nanocarriers of their therapeutic with the MRI contrast gadoteridol for real time treatment imaging.[13] Other than MRI contrasts, it has been shown to be possible to tag a therapeutic microcarrier with a radiolabeled or fluorescent molecule that can then be excited during imaging. The biggest limitation of this drug distribution visualization is that this technique only works ex vivo. One research group was able to optimize their liposomal design using this technique, showing the usefulness of this technique.[14]
While a common use of CED is to directly deliver drugs to the brain, it is also possible to deliver non-chemical therapeutics, such as proteins or growth factors, using CED. There are several types of microcarriers which have been used for CED, including nanospheres, nanoparticles, liposomes, micelles, and dendrites.[15] Nanocarriers have several unique benefits for delivering therapeutics compared to conventional drug solutions. Firstly, nanocarriers can be modified to create an optimal carrier for the system that is being developed. These modifications can include tagging them for imaging, size, charge, osmolarity, viscosity, and changes in surface coating.[15]
The other large area of research being done currently on CED is the translation of CED from being used for brain tumors to other brain diseases. The primary conditions being researched for non-cancerous treatments include Parkinson’s disease and epilepsy. Animal model research using CED to deliver therapeutics to the brain to treat Parkinson’s disease have shown 3 promising therapeutics for treatment. Researchers for these therapeutics have typically used adenovirus carriers for therapeutics since many drugs used to treat Parkinson’s disease currently are not chemical based but rather gene therapy or protein based.[16] Current research areas focus on using GDNF, a growth factor which protects dopamine producing brain cells, glutamic acid decarboxylase (GAD), which is another therapeutic that helps to protect dopamine producing brain cells, and neurturin, which is a GDNF homolog.[16] Another reported use of CED is in the treatment of epilepsy. Current epilepsy treatments are too large to pass through the BBB, so utilizing CED to delivery these drugs is currently one of the only ways to target the brain. The two primary antiepileptic drugs (AEDs) being delivered using CED in research are conotoxin N-type calcium channel antagonists and botulinum neurotoxins.[2] Results from these studies showed promise in reducing the risk of seizures for up to 5 days when treated with calcium channel antagonists and up to 50 days when using botulinum neurotoxins [17][18]
Limitations and future directions
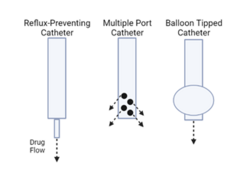
While there has been promise in the use of CED to delivery drugs directly into the brain, there are some drawbacks with it. A vast majority of studies to date have failed to have consistent delivery from patient to patient for technical reasons surrounding the usage of CED. Incorrect placement of catheters can result in a less effective treatment with increased risks of leaks from the brain into other parts of the central nervous system (CNS). Another, more common occurrence is the incidence or reflux of the drug back into the catheter. Reflux can cause leakage into unintended areas as well as decrease the true volume of drug delivered.[6] CED catheter improvements are currently being researched, with some research groups modifying the tips of the catheters to prevent reflux. The design of a balloon tipped catheter for use in CED has been proposed, and results showed that drug was successfully delivered into the brain using the balloon tipped catheter without any complication.[19] Other proposed designs include the utilization of catheters with multiple exit sites, catheters with porous tips, and catheters with tips that are smaller than the rest of the catheter.[20] New catheter designs also aim to allow for a greater flow rate while still minimizing the risk of reflux.[13] These improvements to the technical limitations of CED aim to help researchers determine efficacy of a treatment without worrying about failed treatments due to limitations in the equipment of CED. With this in mind, there is a fast growing tech company in Baltimore, Maryland named CraniUS LLC, for which is inventing, designing and engineering the world's first fully-implantable, MRI-compatible, wirelessly-charged, bluetooth-enabled, high-profile craniofacial implant device to provide neurosurgical patients a safe option for receiving direct and chronic medicine delivery to their brain via convection-enhanced delivery; using a novel embedded, microfluidic-pump system and easy port-access system for repeated, transcutaneous filling. |url=http://www.CraniUSmed.com
References
- ↑ Jahangiri, Arman; Chin, Aaron T.; Flanigan, Patrick M.; Chen, Rebecca; Bankiewicz, Krystof; Aghi, Manish K. (January 2017). "Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies". Journal of Neurosurgery 126 (1): 191–200. doi:10.3171/2016.1.jns151591. ISSN 0022-3085. PMID 27035164. PMC 5571827. http://dx.doi.org/10.3171/2016.1.jns151591.
- ↑ 2.0 2.1 Rogawski, Michael A. (April 2009). "Convection-enhanced delivery in the treatment of epilepsy". Neurotherapeutics 6 (2): 344–351. doi:10.1016/j.nurt.2009.01.017. ISSN 1933-7213. PMID 19332329. PMC 2753495. http://dx.doi.org/10.1016/j.nurt.2009.01.017.
- ↑ Lam, Miu Fei; Thomas, Meghan G.; Lind, Christopher R.P. (September 2011). "Neurosurgical convection-enhanced delivery of treatments for Parkinson's disease". Journal of Clinical Neuroscience 18 (9): 1163–1167. doi:10.1016/j.jocn.2011.01.012. ISSN 0967-5868. PMID 21745745. http://dx.doi.org/10.1016/j.jocn.2011.01.012.
- ↑ Bellettato, Cinzia M.; Scarpa, Maurizio (November 2018). "Possible strategies to cross the blood–brain barrier". Italian Journal of Pediatrics 44 (S2): 131. doi:10.1186/s13052-018-0563-0. ISSN 1824-7288. PMID 30442184.
- ↑ 5.0 5.1 5.2 Mehta, A. M.; Sonabend, A. M.; Bruce, J. N. (2017-03-15). "Convection-Enhanced Delivery". Neurotherapeutics 14 (2): 358–371. doi:10.1007/s13311-017-0520-4. ISSN 1933-7213. PMID 28299724. PMC 5398992. http://dx.doi.org/10.1007/s13311-017-0520-4.
- ↑ 6.0 6.1 Lonser, Russell R.; Sarntinoranont, Malisa; Morrison, Paul F.; Oldfield, Edward H. (March 2015). "Convection-enhanced delivery to the central nervous system". Journal of Neurosurgery 122 (3): 697–706. doi:10.3171/2014.10.jns14229. ISSN 0022-3085. PMID 25397365.
- ↑ Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z. et al. (2010-08-01). "Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma" (in en). Neuro-Oncology 12 (8): 871–881. doi:10.1093/neuonc/nop054. ISSN 1522-8517. PMID 20511192. PMC 2940677. https://academic.oup.com/neuro-oncology/article-lookup/doi/10.1093/neuonc/nop054.
- ↑ Isarna Therapeutics GmbH (2014-11-04). Efficacy and Safety of AP 12009 in Adult Patients With Recurrent or Refractory Anaplastic Astrocytoma or Secondary Glioblastoma as Compared to Standard Chemotherapy Treatment: A Randomized, Actively Controlled, Open Label Clinical Phase III Study.. https://clinicaltrials.gov/ct2/show/study/NCT00761280.
- ↑ National Institute of Neurological Disorders and Stroke (NINDS) (2017-10-05). Convection Enhanced Delivery of Muscimol to Study the Pathophysiology Underlying the Clinical Features of Parkinson's Disease. https://clinicaltrials.gov/ct2/show/study/NCT00921128.
- ↑ "AAV2-GDNF for Advanced Parkinson s Disease - Tabular View - ClinicalTrials.gov" (in en). https://clinicaltrials.gov/ct2/show/record/NCT01621581.
- ↑ Bankiewicz, Krys S.; Eberling, Jamie L.; Kohutnicka, Malgorzata; Jagust, William; Pivirotto, Phillip; Bringas, John; Cunningham, Janet; Budinger, Thomas F. et al. (July 2000). "Convection-Enhanced Delivery of AAV Vector in Parkinsonian Monkeys; In Vivo Detection of Gene Expression and Restoration of Dopaminergic Function Using Pro-drug Approach". Experimental Neurology 164 (1): 2–14. doi:10.1006/exnr.2000.7408. ISSN 0014-4886. PMID 10877910. http://dx.doi.org/10.1006/exnr.2000.7408.
- ↑ Mardor, Yael; Rahav, Ofer; Zauberman, Yacov; Lidar, Zvi; Ocherashvilli, Aharon; Daniels, Dianne; Roth, Yiftach; Maier, Stephan E. et al. (2005-08-01). "Convection-Enhanced Drug Delivery: Increased Efficacy and Magnetic Resonance Image Monitoring". Cancer Research 65 (15): 6858–6863. doi:10.1158/0008-5472.can-05-0161. ISSN 0008-5472. PMID 16061669. http://dx.doi.org/10.1158/0008-5472.can-05-0161.
- ↑ 13.0 13.1 Fiandaca, Massimo S.; Forsayeth, John R.; Dickinson, Peter J.; Bankiewicz, Krystof S. (January 2008). "Image-guided convection-enhanced delivery platform in the treatment of neurological diseases". Neurotherapeutics 5 (1): 123–127. doi:10.1016/j.nurt.2007.10.064. ISSN 1933-7213. PMID 18164491. PMC 2719019. http://dx.doi.org/10.1016/j.nurt.2007.10.064.
- ↑ MacKay, J. Andrew; Deen, Dennis F.; Szoka, Francis C. (February 2005). "Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating". Brain Research 1035 (2): 139–153. doi:10.1016/j.brainres.2004.12.007. ISSN 0006-8993. PMID 15722054. http://dx.doi.org/10.1016/j.brainres.2004.12.007.
- ↑ 15.0 15.1 Allard, Emilie; Passirani, Catherine; Benoit, Jean-Pierre (April 2009). "Convection-enhanced delivery of nanocarriers for the treatment of brain tumors". Biomaterials 30 (12): 2302–2318. doi:10.1016/j.biomaterials.2009.01.003. ISSN 0142-9612. PMID 19168213. http://dx.doi.org/10.1016/j.biomaterials.2009.01.003.
- ↑ 16.0 16.1 Lam, Miu Fei; Thomas, Meghan G.; Lind, Christopher R.P. (September 2011). "Neurosurgical convection-enhanced delivery of treatments for Parkinson's disease". Journal of Clinical Neuroscience 18 (9): 1163–1167. doi:10.1016/j.jocn.2011.01.012. ISSN 0967-5868. PMID 21745745. http://dx.doi.org/10.1016/j.jocn.2011.01.012.
- ↑ Gasior, Maciej; White, Natalie A.; Rogawski, Michael A. (2007-08-23). "Prolonged Attenuation of Amygdala-Kindled Seizure Measures in Rats by Convection-Enhanced Delivery of the N-Type Calcium Channel Antagonists ω-Conotoxin GVIA and ω-Conotoxin MVIIA". Journal of Pharmacology and Experimental Therapeutics 323 (2): 458–468. doi:10.1124/jpet.107.125047. ISSN 0022-3565. PMID 17717191. PMC 2257985. http://dx.doi.org/10.1124/jpet.107.125047.
- ↑ Gasior, Maciej; Tang, Rebecca; Rogawski, Michael A. (2013-06-14). "Long-Lasting Attenuation of Amygdala-Kindled Seizures after Convection-Enhanced Delivery of Botulinum Neurotoxins A and B into the Amygdala in Rats". Journal of Pharmacology and Experimental Therapeutics 346 (3): 528–534. doi:10.1124/jpet.113.205070. ISSN 0022-3565. PMID 23772062. PMC 3876783. http://dx.doi.org/10.1124/jpet.113.205070.
- ↑ Olson, Jeffrey J.; Zhang, Zhaobin; Dillehay, Dirk; Stubbs, James (2008-05-06). "Assessment of a balloon-tipped catheter modified for intracerebral convection-enhanced delivery". Journal of Neuro-Oncology 89 (2): 159–168. doi:10.1007/s11060-008-9612-7. ISSN 0167-594X. PMID 18458816. http://dx.doi.org/10.1007/s11060-008-9612-7.
- ↑ Debinski, Waldemar; Tatter, Stephen B (October 2009). "Convection-enhanced delivery for the treatment of brain tumors". Expert Review of Neurotherapeutics 9 (10): 1519–1527. doi:10.1586/ern.09.99. ISSN 1473-7175. PMID 19831841. PMC 3657605. http://dx.doi.org/10.1586/ern.09.99.
 |
