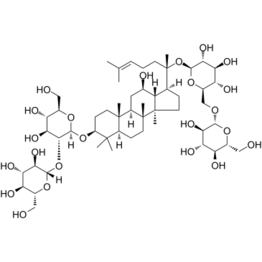
Medicine:Ginsenoside Rb1
Ginsenoside Rb1 is a class steroid found in the extraction of ginseng roots (Panax species) in traditional Asian pharmacology. It belongs to the class of natural product steroid glycosides and triterpene saponins. Ginsenoside Rb1 and Ginsenoside Rg1 are two of the most-used components in Panax notoginseng saponins. The ginsenosides group has a variety of potential health benefits including anti-carcinogenic, anti‐inflammatory, anti-allergic, anti-atherosclerotic, antihypertensive, and anti-diabetic effects. It also provides anti-stress properties on the central nervous system.[1]
Pharmacological effects
Research suggests that Ginsenoside Rg1(GRg1) and Ginsenoside Rb1(GRb1) offer protection against symptoms of Alzheimer's disease in mice. The GRg1 affects three metabolic pathways in mice with Alzheimer's: the metabolism of lecithin, amino acids, and hospholipase. GRb1 treatment affects lecithin and amino acid metabolism.[2][3]
In 1998, the Oh group at Seoul National University, South Korea, reported that GRb1 and Grg3 significantly attenuated amalgamate-induced neuroticism by inhibiting the overproduction of nitric oxide, among some other findings regarding their neuron-protective property.[4] In 2002, the Laboratory for Cancer Research at Rutgers University showed that GRb1 and GRg1 had a neuroprotective effect on spinal cord neurons, while Ginsenoside Re did not exhibit any activity. The research group concluded that Ginsenosides Rb1 and Rg1 comprise potentially-effective therapeutic agents for spinal cord injuries.[5]
It is reported that Ginsenoside Rb1 improves cardiac function and remodeling in heart failure in mice.[6] The treatment of H-ginsenoside Rb1 potentially attenuates cardiac hypertrophy and myocardial fibrosis.
Biosynthesis
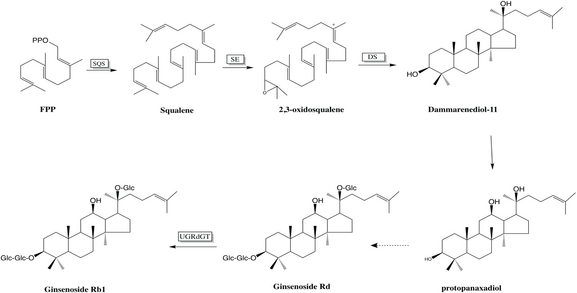
The biosynthesis of Ginsenoside Rb1 in Panax ginseng[7] starts from FPP, which presumably is synthesized from IPP and DMAPP. The FPP is converted to squalene by squalene synthase (SQS) in the first step of the mechanism. One dimethyl substituted alkene was converted to epoxide by squalene epoxidase (SE). The 2,3-oxidasqualene was converted to dammarenediol-II by dammarenediol-II synthase(DS) presumably by polycyclization hydration reaction. In the last synthetic step, ginsenoside Rb1 was synthesized from ginsenoside Rd by ginsenoside Rd glucosyltransferase (UGRdGT),[8][9] a new biosynthetic enzyme of ginsenoside Rb1.
References
- ↑ Christensen, L. P. (2008). "Chapter 1 Ginsenosides: Chemistry, Biosynthesis, Analysis, and Potential Health Effects". Advances in Food and Nutrition Research 55: 1-99. https://doi.org/10.1016/S1043-4526(08)00401-4.
- ↑ Li, Naijing; Zhou, Ling; Li, Wei; Liu, Ying; Wang, Jiahe; He, Ping (March 2015). "Protective effects of ginsenosides Rg1 and Rb1 on an Alzheimer's disease mouse model: A metabolism study" (in en). Journal of Chromatography B 985: 54–61. doi:10.1016/j.jchromb.2015.01.016. https://linkinghub.elsevier.com/retrieve/pii/S1570023215000495.
- ↑ Ulla Holtbäck, Anita C. Aperia, in Seldin and Giebisch's The Kidney (Fourth Edition), 2008
- ↑ Kim, Young C.; Kim, So R.; Markelonis, George J.; Oh, Tae H. (1998). "Ginsenosides Rb1and Rg3protect cultured rat cortical cells from glutamate-induced neurodegeneration" (in en). Journal of Neuroscience Research 53 (4): 426–432. doi:10.1002/(SICI)1097-4547(19980815)53:43.0.CO;2-8. ISSN 1097-4547. https://onlinelibrary.wiley.com/doi/abs/10.1002/%28SICI%291097-4547%2819980815%2953%3A4%3C426%3A%3AAID-JNR4%3E3.0.CO%3B2-8.
- ↑ Liao, Baisong; Newmark, Harold; Zhou, Renping (February 2002). "Neuroprotective Effects of Ginseng Total Saponin and Ginsenosides Rb1 and Rg1 on Spinal Cord Neurons in Vitro" (in en). Experimental Neurology 173 (2): 224–234. doi:10.1006/exnr.2001.7841. https://linkinghub.elsevier.com/retrieve/pii/S0014488601978410.
- ↑ Zheng, Xian; Wang, Shuai; Zou, Xiaoming; Jing, Yating; Yang, Ronglai; Li, Siqi; Wang, Fengrong (2017). "Ginsenoside Rb1 improves cardiac function and remodeling in heart failure" (in en). Experimental Animals 66 (3): 217–228. doi:10.1538/expanim.16-0121. ISSN 1341-1357. PMID 28367863. PMC 5543242. https://www.jstage.jst.go.jp/article/expanim/66/3/66_16-0121/_article.
- ↑ 7.0 7.1 Huang, Chao; Zhong, Jian-Jiang (2013-08-01). "Elicitation of ginsenoside biosynthesis in cell cultures of Panax ginseng by vanadate" (in en). Process Biochemistry 48 (8): 1227–1234. doi:10.1016/j.procbio.2013.05.019. ISSN 1359-5113. http://www.sciencedirect.com/science/article/pii/S1359511313002663.
- ↑ Yue, Cai-Jun; Zhong, Jian-Jiang (2005-02-20). "Impact of external calcium and calcium sensors on ginsenoside Rb1 biosynthesis byPanax notoginseng cells" (in en). Biotechnology and Bio-engineering 89 (4): 444–452. doi:10.1002/bit.20386. ISSN 0006-3592. http://doi.wiley.com/10.1002/bit.20386.
- ↑ Zhong, Jian-Jiang; Yue, Cai-Jun (2005), Nielsen, J., ed., "Plant Cells: Secondary Metabolite Heterogeneity and Its Manipulation" (in en), Biotechnology for the Future, Advances in Biochemical Engineering/Biotechnology (Springer): pp. 53–88, doi:10.1007/b136412, ISBN 978-3-540-31554-4, https://doi.org/10.1007/b136412, retrieved 2020-06-07
External Link
 |