Medicine:Open flow microperfusion
Open flow microperfusion (OFM) is a sampling method for clinical and preclinical drug development studies and biomarker research. OFM is designed for continuous sampling of analytes from the interstitial fluid (ISF) of various tissues. It provides direct access to the ISF by insertion of a small, minimally invasive, membrane-free probe with macroscopic openings.[1] Thus, the entire biochemical information of the ISF becomes accessible regardless of the analyte's molecular size, protein-binding property or lipophilicity. OFM is capable of sampling lipophilic and hydrophilic compounds,[2] protein bound and unbound drugs,[3][4] neurotransmitters, peptides and proteins, antibodies,[5][6][7] nanoparticles and nanocarriers, enzymes and vesicles.
Method
The OFM probes are perfused with a physiological solution (the perfusate) which equilibrates with the ISF of the surrounding tissue. Operating flow rates range from 0.1 to 10 μL/min. OFM allows unrestricted exchange of compounds via an open structure across the open exchange area of the probe. This exchange of compounds between the probe’s perfusate and the surrounding ISF is driven by convection and diffusion, and occurs non-selectively in either direction (Figure 1).
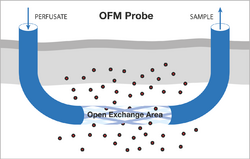
The direct liquid pathway between the probe’s perfusate and the surrounding fluid results in collection of ISF samples. These samples can be collected frequently and are then subjected to bioanalytical analysis to enable monitoring of substance concentrations with temporal resolution during the whole sampling period.[8][9][10]
History
The first OFM sampling probe was described in an Austrian patent application filed by Falko Skrabal in 1987, where OFM was described as a device, which can be implanted into the tissue of living organisms.[11] In 1992, a US patent was filed claiming a device for determining at least one medical variable in the tissue of living organisms.[12] In a later patent by Helmut Masoner, Falko Skrabal and Helmut List a linear type of the sampling probe with macroscopic circular holes was also disclosed.[13] Alternative and current OFM versions for dermal and adipose tissue application were developed by Joanneum Research, and were patented by Manfred Bodenlenz et al.[14][15] Alternative materials featuring low absorption were used to enable manufacturing of probes with diameters of 0.55 mm and exchange areas of 15 mm in length. For cerebral application, special OFM probes were patented by Birngruber et al.[16] Additionally, a patent was filed to manage the fluid handling of the ISF by using a portable peristaltic pump with a flow range of 0.1 to 10 µL/min that enables operation of up to three probes per pump.[17]
OFM System
Two types of OFM probes are currently available: Linear OFM probes for implantation into superficial tissues such as skin (dermal OFM, dOFM) and subcutaneous adipose tissue (adipose OFM, aOFM) as well as concentric probes for implantation into various regions of the brain (cerebral OFM, cOFM).
Areas of application
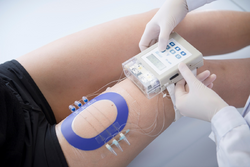
OFM is routinely applied in pharmaceutical research in preclinical (e.g. mice, rats, pigs, primates) and in clinical studies in humans (Figure 2). OFM-related procedures such as probe insertions or prolonged sampling with numerous probes are well tolerated by the subjects.[18]
Dermal OFM (dOFM)
dOFM (Figure 3) allows the investigation of transport of drugs in the dermis and their penetration into the dermis after local, topical or systemic application, and dOFM is recommended by the U.S. Food and Drug Administration as a new method for assessment of bioequivalence of topical drugs.[19][20][21]
[BB1]Hier Verlinkung zur FDA Seite
- conduct tissue-specific pharmacokinetic (PK) and pharmacodynamic (PD) studies of drugs.[22][23]
- perform head-to-head comparison of novel topical drug formulations
- assess dermal bioavailability.[24][25]
- investigate high molecular weight compounds, e.g. antibodies[26]
Head-to-head settings with OFM have proven particularly useful for the evaluation of topical generic products, which need to demonstrate bioequivalence[27] to the reference listed drug product to obtain market approval.
Applications of dOFM include ex vivo studies with tissue explants and preclinical and clinical in vivo studies.

Adipose OFM (aOFM)
aOFM (Figure 3) allows continuous on-line monitoring of metabolic processes in the subcutaneous adipose tissue, e.g. glucose and lactate,[28][29][30] as well as larger analytes such as insulin (5.9 kDa).[31][32] The role of polypeptides for metabolic signaling (leptin, cytokine IL-6, TNFα) has also been studied with aOFM.[33] aOFM allows the quantification of proteins (e.g. albumin size: 68 kDa) in adipose tissue[34] and thus opens up the possibility to investigate protein-bound drugs directly in peripheral target tissues, such as highly protein-bound insulin analogues designed for a prolonged, retarded insulin action.[35] Most recently, aOFM has been used to sample agonists to study obesity, lipid metabolism and immune-inflammation. Applications of aOFM include ex vivo studies with tissue explants and preclinical and clinical in vivo studies.
Cerebral OFM (cOFM)
cOFM (Figure 4) is used to conduct PK/PD preclinical studies in the animal brain. Access to the brain includes monitoring of the blood-brain barrier function and drug transport across the intact blood-brain barrier.[36] cOFM allows taking a look behind the blood-brain barrier and assesses concentrations and effects of neuroactive substances directly in the targeted brain tissue.[37]
The blood-brain barrier is a natural shield that protects the brain and limits the exchange of nutrients, metabolites and chemical messengers between blood and brain. The blood-brain barrier also prevents potential harmful substances from entering and damaging the brain. However, this highly effective barrier also prevents neuroactive substances from reaching appropriate targets. For researchers that develop neuroactive drugs, it is therefore of major interest to know whether and to what extent an active pharmaceutical component can pass the blood-brain barrier. Experiments have shown that the blood-brain barrier has fully reestablished 15 days after implantation of the cOFM probe in the brain of rats.[38] The cOFM probe has been specially designed to avoid a reopening of the blood-brain barrier or causing additional trauma to the brain after implantation. cOFM enables continuous sampling of cerebral ISF with intact blood-brain barrier cOFM and thus allows continuous PK monitoring in brain tissue.
Quantification of ISF compounds
ISF compounds can be quantified either indirectly from merely diluted ISF samples by using OFM and additional calibration techniques, or directly from undiluted ISF samples which can be collected with additional OFM methods. Quantification of compounds from diluted ISF samples requires additional application of calibration methods, such as Zero Flow Rate,[39] No Net Flux[40] or Ionic Reference.[41] Zero Flow Rate has been used in combination with dOFM by Schaupp et al.[42] to quantify potassium, sodium and glucose in adipose ISF samples. No Net Flux has been applied to quantify several analytes in OFM studies in subcutaneous adipose, muscle and dermal ISF: the absolute lactate concentrations[43] and the absolute glucose concentrations in adipose ISF,[44] the absolute albumin concentration in muscle ISF[45] and the absolute insulin concentration in adipose and muscle ISF have been successfully determined.[46] Dragatin et al.[47] used No Net Flux in combination with dOFM to assess the absolute ISF concentration of a fully human therapeutic antibody. Ionic Reference has been used in combination with OFM to assess the absolute glucose concentration[48] and the absolute lactate concentration in adipose ISF.[49] Dermal OFM has also been used to quantify the concentrations of human insulin and an insulin analogue in the ISF with inulin as exogenous marker.
Additional OFM methods, such as OFM recirculation and OFM suction can collect undiluted ISF samples from which direct and absolute quantification of compounds is feasible.[50] OFM recirculation to collect undiluted ISF samples recirculates the perfusate in a closed loop until equilibrium concentrations between perfusate and ISF are established. Using albumin as analyte, 20 recirculation cycles have been enough to reach equilibrium ISF concentrations. OFM suction is performed by applying a mild vacuum, which pulls ISF from the tissue into the OFM probe.
References
- ↑ Bodenlenz M, Aigner B, Dragatin C, Liebenberger L, Zahiragic S, Höfferer C, et al. Clinical applicability of dOFM devices for dermal sampling. Ski Res Technol Technol. 2013 Nov;19(4):474–83.
- ↑ Altendorfer-Kroath, T.; Schimek, D.; Eberl, A.; Rauter, G.; Ratzer, M.; Raml, R.; Sinner, F. M.; Birngruber, T., 2019: Comparison of cerebral Open Flow Microperfusion and Microdialysis when sampling small lipophilic and small hydrophilic substances. Journal of Neuroscience Methods., 311, 394–401.
- ↑ Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, et al. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol. 1999;276(2):401–8.
- ↑ Ellmerer M, Schaupp L, Brunner GA, Sendlhofer G, Wutte A, Wach P, et al. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am J Physiol - Endocrinol Metab. 2000 Feb;278(2):352–6.
- ↑ Dragatin C, Polus F, Bodenlenz M, Calonder C, Aigner B, Tiffner KI, et al. Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp Dermatol. 2016 Feb;25(2):157–9
- ↑ Kolbinger F, Loesche C, Valentin M-A, Jiang X, Cheng Y, Jarvis P, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017 Mar 5;139(3):923–32
- ↑ Kleinert, M.; Kotzbeck, P.; Altendorfer-Kroath, T.; Birngruber, T.; Tschöp, M. H.; Clemmensen, C., 2018: Time-resolved hypothalamic open flow micro-perfusion reveals normal leptin transport across the blood–brain barrier in leptin resistant mice. Molecular Metabolism., 13, 77–82.
- ↑ Bodenlenz M, Dragatin C, Liebenberger L, Tschapeller B, Boulgaropoulos B, Augustin T, et al. Kinetics of Clobetasol-17-Propionate in Psoriatic Lesional and Non-Lesional Skin Assessed by Dermal Open Flow Microperfusion with Time and Space Resolution. Pharm Res 2016;33:2229–38.
- ↑ Bodenlenz M, Tiffner KI, Raml R, Augustin T, Dragatin C, Birngruber T, et al. Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Clin Pharmacokinet. 2017 Jan;56(1).
- ↑ Bodenlenz M, Tiffner KI, Raml R, Augustin T, Dragatin C, Birngruber T, et al. Erratum to: Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Clin Pharmacokinet [Internet]. 2017 Jan;56(1):99. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27873172
- ↑ F. Skrabal, Vorrichtung zur Bestimmung zumindestens einer medizinischen Substanz in lebenden Organismen, AT391998B, 1987
- ↑ F. Skrabal, Process for determining parameters of interest in living organisms, 5,097,834, 1992.
- ↑ H. Marsoner, F. Skrabal, H. List, Device for determining at least one medical variable, 5,193,545, 1993.
- ↑ M. Bodenlenz, L. Schaupp, Catheter having an oblong slit, WO 2007/131780 A1, 2007.
- ↑ M. Bodenlenz, C. Hoefferer, T. Birngruber, L. Schaupp, Filament-based catheter, WO 2010/031515, 2010
- ↑ WO2012156478A1
- ↑ M. Bodenlenz, C. Hoefferer, T. Birngruber, R. Schaller, J. Priedl, F. Feichtner, S. Schock, P. Tkaczyk, and L. Schaupp, “Pump for medical applications” DE102011090210A1, 2011. EP;US;CA; 2011. Patent pending.
- ↑ M. Bodenlenz, B. Aigner, C. Dragatin, L. Liebenberger, S. Zahiragic, C. Höfferer, T. Birngruber, J. Priedl, F. Feichtner, L. Schaupp, S. Korsatko, M. Ratzer, C. Magnes, T.R. Pieber, F. Sinner, Clinical applicability of dOFM devices for dermal sampling. Skin Res. Technol. 19, 474–483 (2013).
- ↑ (in en) A New Possible Way to Evaluate Bioequivalence of Topical Drugs, https://www.youtube.com/watch?v=MQAaOWo0NIo, retrieved 2021-05-03
- ↑ Research, Center for Drug Evaluation and (2021-01-28). "Impact Story: Developing New Ways to Evaluate Bioequivalence for Topical Drugs" (in en). FDA. https://www.fda.gov/drugs/regulatory-science-action/impact-story-developing-new-ways-evaluate-bioequivalence-topical-drugs.
- ↑ "OGD_GDUFA_ScienceandResearchReport_FINAL_210402.pdf". https://www.fda.gov/media/146749/download#page=122.
- ↑ M. Bodenlenz, C. Höfferer, C. Magnes, R. Schaller-Ammann, L. Schaupp, F. Feichtner, M. Ratzer, K. Pickl, F. Sinner, A. Wutte, S. Korsatko, G. Köhler, F. J. Legat, E. M. Benfeldt, A. M. Wright, D. Neddermann, T. Jung, and T. R. Pieber, “Dermal PK/PD of a lipophilic topical drug in psoriatic patients by continuous intradermal membrane-free sampling.,” Eur. J. Pharm. Biopharm., vol. 81, no. 3, pp. 635–41, Aug. 2012. [10]
- ↑ Bodenlenz M, Höfferer C, Magnes C, Schaller-Ammann R, Schaupp L, Feichtner F, et al. Dermal PK/PD of a lipophilic topical drug in psoriatic patients by continuous intradermal membrane-free sampling. Eur J Pharm Biopharm. 2012 Aug;81(3):635–41.
- ↑ Bodenlenz M, Tiffner KI, Raml R, Augustin T, Dragatin C, Birngruber T, et al. Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Clin Pharmacokinet. 2017 Jan;56(1):91–8.
- ↑ M. Bodenlenz, B. Aigner, C. Dragatin, L. Liebenberger, S. Zahiragic, C. Höfferer, T. Birngruber, J. Priedl, F. Feichtner, L. Schaupp, S. Korsatko, M. Ratzer, C. Magnes, T.R. Pieber, F. Sinner, Clinical applicability of dOFM devices for dermal sampling. Skin Res. Technol. 19, 474–483 (2013).
- ↑ Dragatin C, Polus F, Bodenlenz M, Calonder C, Aigner B, Tiffner KI, et al. Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp Dermatol. 2016 Feb;25(2):157–9.
- ↑ Bodenlenz M, Tiffner KI, Raml R, Augustin T, Dragatin C, Birngruber T, et al. Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence. Clin Pharmacokinet. 2017 Jan;56(1):91–8.
- ↑ F. Skrabal, Z. Trajanoski, H. Kontscheider, P. Kotanko, P. Wach, Portable system for on-line continuous ex vivo monitoring of subcutaneous tissue glucose using open tissue perfusion. Med. Biol. Eng. Comput. 33, 116–8 (1995).
- ↑ M. Ellmerer, L. Schaupp, Z. Trajanoski, G. Jobst, I. Moser, G. Urban, F. Skrabal, P. Wach, Continuous measurement of subcutaneous lactate concentration during exercise by combining open-flow microperfusion and thin-film lactate sensors. Biosens. Bioelectron. 13, 1007–13 (1998).
- ↑ G.W. Cline, K.F. Petersen, M. Krssak, J. Shen, R.S. Hundal, Z. Trajanoski, S. Inzucchi, A. Dresner, D.L. Rothman, G.I. Shulman, Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–6 (1999).
- ↑ W. Cline, B.M. Jucker, Z. Trajanoski, A.J. Rennings, G.I. Shulman, A novel 13C NMR method to assess intracellular glucose concentration in muscle, in vivo. Am. J. Physiol. 274, E381-9 (1998).
- ↑ G.W. Cline, K.F. Petersen, M. Krssak, J. Shen, R.S. Hundal, Z. Trajanoski, S. Inzucchi, A. Dresner, D.L. Rothman, G.I. Shulman, Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N. Engl. J. Med. 341, 240–6 (1999).
- ↑ Orban, A.T. Remaley, M. Sampson, Z. Trajanoski, G.P. Chrousos, The differential effect of food intake and beta-adrenergic stimulation on adipose-derived hormones and cytokines in man. J. Clin. Endocrinol. Metab. 84, 2126–33 (1999).
- ↑ M. Ellmerer, L. Schaupp, G.A. Brunner, G. Sendlhofer, A. Wutte, P. Wach, T.R. Pieber, Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am. J. Physiol. Endocrinol. Metab. 278, E352-6 (2000).
- ↑ Bodenlenz, M.; Ellmerer, M.; Schaupp, L.; Jacobsen, L. V.; Plank, J.; Brunner, G. A.; Wutte, A.; Aigner, B. et al. (2015). "Bioavailability of insulin detemir and human insulin at the level of peripheral interstitial fluid in humans, assessed by open-flow microperfusion". Diabetes, Obesity and Metabolism 17 (12): 1166–1172. doi:10.1111/dom.12551. PMID 26260082.
- ↑ Birngruber, Thomas; Sinner, Frank (2016). "Cerebral open flow microperfusion (COFM) an innovative interface to brain tissue". Drug Discovery Today: Technologies 20: 19–25. doi:10.1016/j.ddtec.2016.07.003. PMID 27986219.
- ↑ Birngruber, Thomas; Raml, Reingard; Gladdines, Werner; Gatschelhofer, Christina; Gander, Edgar; Ghosh, Arijit; Kroath, Thomas; Gaillard, Pieter J. et al. (2014). "Enhanced Doxorubicin Delivery to the Brain Administered Through Glutathione PEGylated Liposomal Doxorubicin (2B3-101) as Compared with Generic Caelyx,®/Doxil®—A Cerebral Open Flow Microperfusion Pilot Study". Journal of Pharmaceutical Sciences 103 (7): 1945–1948. doi:10.1002/jps.23994. PMID 24801480.
- ↑ Birngruber, Thomas; Ghosh, Arijit; Perez-Yarza, Veronica; Kroath, Thomas; Ratzer, Maria; Pieber, Thomas R.; Sinner, Frank (2013). "Cerebral open flow microperfusion: A newin vivotechnique for continuous measurement of substance transport across the intact blood-brain barrier". Clinical and Experimental Pharmacology and Physiology 40 (12): 864–871. doi:10.1111/1440-1681.12174. PMID 24256164.
- ↑ Jacobson I, Sandberg M HA. Mass transfer in brain dialysis devices--a new method for the estimation of extracellular amino acids concentration. J Neurosci Methods. 1985;15(3):263–8.
- ↑ Löhnroth P, Jansson P, Smith U. A microdialysis method allowing characterization of extracellular water space in humans. Am J Physiol. 1987;253:228–31
- ↑ Trajanoski Z, Brunner G, Schaupp L, Ellmerer M, P W, Pieber T, et al. Open-flow microperfusion of subcutaneous adipose tissue for on-line continuous ex vivo measurement of glucose concentration. Diabetes Care. 1997;20(7):1114–21.
- ↑ Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, et al. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol. 1999;276(2):401–8.
- ↑ Ellmerer M, Schaupp L, Sendlhofer G, Wutte A, Brunner GA, Trajanoski Z, et al. Lactate metabolism of subcutaneous adipose tissue studied by open flow microperfusion. J Clin Endocrinol Metab. 1998 Dec;83(12):4394–401
- ↑ Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, et al. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol. 1999;276(2):401–8.
- ↑ Ellmerer M, Schaupp L, Brunner GA, Sendlhofer G, Wutte A, Wach P, et al. Measurement of interstitial albumin in human skeletal muscle and adipose tissue by open-flow microperfusion. Am J Physiol - Endocrinol Metab. 2000 Feb;278(2):352–6.
- ↑ Bodenlenz M, Schaupp L a, Druml T, Sommer R, Wutte A, Schaller HC, et al. Measurement of interstitial insulin in human adipose and muscle tissue under moderate hyperinsulinemia by means of direct interstitial access. Am J Physiol Endocrinol Metab. 2005 Aug;289(2):296–300.
- ↑ Dragatin C, Polus F, Bodenlenz M, Calonder C, Aigner B, Tiffner KI, et al. Secukinumab distributes into dermal interstitial fluid of psoriasis patients as demonstrated by open flow microperfusion. Exp Dermatol. 2016 Feb;25(2):157–9.
- ↑ Schaupp L, Ellmerer M, Brunner GA, Wutte A, Sendlhofer G, Trajanoski Z, et al. Direct access to interstitial fluid in adipose tissue in humans by use of open-flow microperfusion. Am J Physiol. 1999;276(2):401–8.
- ↑ Ellmerer M, Schaupp L, Sendlhofer G, Wutte A, Brunner GA, Trajanoski Z, et al. Lactate metabolism of subcutaneous adipose tissue studied by open flow microperfusion. J Clin Endocrinol Metab. 1998 Dec;83(12):4394–401.
- ↑ Hummer, Joanna; Schwingenschuh, Simon; Raml, Reingard; Boulgaropoulos, Beate; Schwagerle, Gerd; Augustin, Thomas; Sinner, Frank; Birngruber, Thomas (2020-10-21). "OFM recirculation and OFM suction: Advanced in-vivo open flow microperfusion methods for direct and absolute quantification of albumin in interstitial fluid". Biomedical Physics & Engineering Express. doi:10.1088/2057-1976/abc3a7. ISSN 2057-1976. https://iopscience.iop.org/article/10.1088/2057-1976/abc3a7.
 |
