Philosophy:Mitochondrial replacement therapy
| Mitochondrial replacement therapy | |
|---|---|
| Other names | Mitochondrial donation |
| MeSH | D000069321 |
Mitochondrial replacement therapy (MRT), sometimes called mitochondrial donation, is the replacement of mitochondria in one or more cells to prevent or ameliorate disease. MRT originated as a special form of in vitro fertilisation in which some or all of the future baby's mitochondrial DNA (mtDNA) comes from a third party. This technique is used in cases when mothers carry genes for mitochondrial diseases. The therapy is approved for use in the United Kingdom.[1][2] A second application is to use autologous mitochondria to replace mitochondria in damaged tissue to restore the tissue to a functional state. This has been used in clinical research in the United States to treat cardiac-compromised newborns.[3]
Medical uses
In vitro fertilisation
Mitochondrial replacement therapy has been used to prevent the transmission of mitochondrial diseases from mother to child; it could only be performed in clinics licensed by the UK's Human Fertilisation and Embryology Authority (HFEA), only for people individually approved by the HFEA, for whom preimplantation genetic diagnosis is unlikely to be helpful, and only with informed consent that the risks and benefits are not well understood.[4]
Relevant mutations are found in about 0.5% of the population and disease affects around one in 5000 individuals (0.02%)—the percentage of people affected is much smaller because cells contain many mitochondria, only some of which carry mutations, and the number of mutated mitochondria need to reach a threshold in order to affect the entire cell, and many cells need to be affected for the person to show disease.[2]
The average number of births per year among women at risk for transmitting mtDNA disease is estimated to approximately 150 in the United Kingdom and 800 in the United States .[5]
Prior to the development of MRT, and in places where it is not legal or feasible, the reproductive options for women who are at risk for transmitting mtDNA disease and who want to prevent transmission were using an egg from another woman, adoption, or childlessness.[1]:45
Tissue function
Autologous mitochondria extracted from healthy tissue and supplied to damaged tissue has been used to treat cardiac-compromised newborns. Alternatives to the approach include use of an extracorporeal membrane oxygenator (ECMO) or tissue or organ transplantation.[3]
Techniques
In vitro fertilization involves removing eggs from a woman, collecting sperm from a man, fertilizing the egg with the sperm, allowing the fertilized egg to form a blastocyst, and then transferring the blastocyst into the uterus. MRT involves an additional egg from a third person, and manipulation of both the recipient egg and the donor egg.[citation needed]
As of 2016 there were three MRT techniques in use: maternal spindle transfer (MST); pronuclear transfer (PNT); and the newest technique, polar body transfer (PBT). The original technique, in which mitochondria-containing cytoplasm taken from a donor egg is simply injected into the recipient egg, is no longer used.[1]:46–47
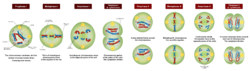
In maternal spindle transfer, an oocyte is removed from the recipient, and when it is in the metaphase II stage of cell division, the spindle-chromosome complex is removed; some of the cytoplasm comes with it, so some mitochondria are likely to be included. The spindle-chromosome complex is inserted into a donor oocyte from which the nucleus has already been removed. This egg is fertilized with sperm and allowed to form a blastocyst, which can then be investigated with preimplantation genetic diagnosis to check for mitochondrial mutations, prior to being implanted in the recipient's uterus.[1]:47–48
In pronuclear transfer, an oocyte is removed from the recipient and fertilized with sperm. The donor oocyte is fertilized with sperm from the same person. The male and female pronuclei are removed from each fertilized egg prior to their fusing, and the pronuclei from the recipient's fertilized egg are inserted into the fertilized egg from the donor. As with MST, a small amount of cytoplasm from the recipient egg may be transferred, and as with MST, the fertilized egg is allowed to form a blastocyst, which can then be investigated with preimplantation genetic diagnosis to check for mitochondrial mutations before being implanted in the recipient's uterus.[1]:50
In polar body transfer, a polar body (a small cell with very little cytoplasm that is created when an egg cell divides) from the recipient is used in its entirety, instead of using nuclear material extracted from the recipient's normal egg; this can be used in either MST or PNT. This technique was first published in 2014 and as of 2015 it had not been consistently replicated, but is considered promising as there is a greatly reduced chance for transmitting mitochondria from the recipient because polar bodies contain very few mitochondria, and it does not involve extracting material from the recipient's egg.[6]

Cytoplasmic transfer
Cytoplasmic transfer was originally developed in the 1980s in the course of basic research conducted with mice to study the role that parts of the cell outside of the nucleus played in embryonic development.[2] In this technique, cytoplasm, including proteins, messenger RNA (mRNA), mitochondria and other organelles, is taken from a donor egg and injected into the recipient egg, resulting in a mixture of mitochondrial genetic material.[2] This technique started to be used in the late 1990s to "boost" the eggs of older women who were having problems conceiving and led to the birth of about 30 babies.[2] Concerns were raised that the mixture of genetic material and proteins could cause problems with respect to epigenetic clashes, or differences in the ability of the recipient and donor materials to effect the development process, or due to the injection of the donor material.[2] After three children born through the technique were found to have developmental disorders (two cases of Turner's syndrome and one case of pervasive developmental disorder (an autism spectrum disorder), the FDA banned the procedure until a clinical trial could prove its safety.[2] As of 2015 that study had not been conducted, but the procedure was in use in other countries.[2]
A related approach uses autologous mitochondria taken from healthy tissue to replace the mitochondria in damaged tissue. Transfer techniques include direct injection into damaged tissue and injection into vessels that supply blood to the tissue.[3]
Risks
Assisted reproduction via MRT involves preimplantation genetic screening of the mother, preimplantation genetic diagnosis after the egg is fertilized, and in vitro fertilization. It has all the risks of those procedures.[1]:60
In addition, both procedures used in MRT entail their own risks. On one level, the procedures physically disrupt two oocytes, removing nuclear genetic material from the recipient egg or fertilized egg and inserting the nuclear genetic material into the donor unfertilized or fertilized egg; the manipulations for both procedures may cause various forms of damage that were not well understood as of 2016.[7]:23
Maternal mitochondria will be carried over to the donor egg; as of 2016 it was estimated that using techniques current in the UK, maternal mitochondria will comprise only around 2% or less of mitochondria in the resulting egg, a level that was considered safe by the HFEA and within the limits of mitochondrial variation that most people have.[7]:23–24
Because MRT procedures involve actions at precise times during egg development and fertilization, and involves manipulating eggs, there is a risk that eggs may mature abnormally or that fertilization may happen abnormally; as of 2016 the HFEA judged that laboratory techniques in the UK had been well enough developed to manage these risks to proceed cautiously with making MRT available.[7]:33–34
Because mitochondria in the final egg will come from a third party, different from the two parties whose DNA is in the nucleus, and because nuclear DNA encodes genes that make some of the proteins and mRNA used by mitochondria, there is a theoretical risk of adverse "mito–nuclear" interactions. While this theoretical risk could possibly be managed by attempting to match the haplotype of the donor and the recipient, as of 2016 there was no evidence that this is an actual risk.[7]:34–37
Because MRT is a relatively new technology, there are concerns that it is not yet safe for public use as there have been limited studies that used MRT in large animal models.[8]
Finally, there is a risk of epigenetic modification to DNA in the nucleus and mitochondria, caused by the procedure itself or by mito–nuclear interactions. As of 2016 these risks appeared to be minimal but were being monitored by long-term study of children born from the procedure.[7]:38
History
In the United States in 1996 embryologist Jacques Cohen and others at the Institute for Reproductive Medicine and Science, Saint Barnabas Medical Center in Livingston, New Jersey first used cytoplasmic transfer in a human assisted reproduction procedure.[9] In 1997 the first baby was born using this procedure. In 2001, Cohen and others reported that ten single babies, twins, and a quadruplet at his New Jersey clinic and a further six children in Israel had been born using his technique. Using modifications of his procedure, a baby had been born at Eastern Virginia Medical School, five children at the Lee Women's Hospital Infertility Clinic in Taichung, Taiwan.[10] twins in Naples, Italy[11] and a twins in India.[12] In total as of 2016, 30–50 children worldwide had been reported to have been born using cytoplasmic transfer.[13]
In 2002, the US Food and Drug Administration (FDA) asked a Biological Response Modifiers Advisory Committee Meeting to advise on the technique of cytoplasmic transfer to treat infertility. This committee felt that there were risks at the time of inadvertent transfer of chromosomes and enhanced survival of abnormal embryos.[13] The FDA informed clinics that they considered the cytoplasmic transfer technique as a new treatment, and, as such, it would require an Investigational New Drug (IND) application. Cohen's clinic started the pre-IND application, but the clinic then went private, funding for the application dried up, the application was abandoned, the research team disbanded,[14] and the cytoplasmic transfer procedure fell out of favor.[15] In 2016, 12 (out of the 13) parents of children born using cytoplasmic transfer at the Saint Barnabas Center participated in a limited follow-up inquiry via online questionnaire. Children whose ages then were 13–18 reported no major problems.[16]
In 2009, a team in Japan published studies of mitochondrial donation.[17] In the same year, a team led by scientists at Oregon Health & Science University published results of mitochondrial donation in monkeys; that team published an update reporting on the health of the monkeys born with the technique, as well as further work it had done on human embryos.[18]
Human trials with oocytes in 2010 by Craven et al. were successful in reducing transmission of mutated mtDNA. The results of the study found the mean transfer DNA (tDNA) carryover to stay under 2% in all of the experimental embryos. This was true for both the MI-SCC and PN transfer methods of MTR. This research did not extend past the blastocyst stage because of ethical concerns, and there are still concerns about whether results retrieved from the blastocyst stage are viable representations of whole embryos. Because of these speculations and to further the viability of MTR as a safe and effective technique, further research and clinical trials would need to be initiated to test the efficacy of MTR in the long term in human patients.[19]
Research in the United Kingdom
In the United Kingdom, following animal experiments and the recommendations of a government commissioned expert committee,[20] the Human Fertilisation and Embryology (Research Purposes) Regulations were passed in 2001 regulating and allowing research into human embryos. In 2004, Newcastle University applied for a license to develop pronuclear transfer to avoid the transmission of mitochondrial diseases,[21] and was granted the license in 2005. Following further research by Newcastle and the Wellcome Trust,[22][23] scientific review,[24] public consultations, and debate, the UK government recommended that mitochondrial donation be legalized in 2013.[25] In 2015 parliament passed the Human Fertilisation and Embryology (Mitochondrial Donation) Regulations, which came into force on 29 October 2015, making human mitochondrial donation legal in the UK. The Human Fertilisation and Embryology Authority (HFEA) was authorized to license and regulate medical centers which wanted to use human mitochondrial donation.[26][27] In February 2016, the US National Academy of Sciences issued a report describing technologies then current and the surrounding ethical issues.[1]
The HFEA Safety Committee issued its fourth report in November 2016 recommending procedures under which HFEA should authorize MRT,[7] the HFEA issued their regulations in December 2016[27][4] and granted their first license (to Newcastle Fertility Centre; Newcastle upon Tyne Hospital NHS Foundation Trust lead by Dr Jane Stewart as Person Responsible to the HFEA) in March 2017.[28] Between August 2017 and January 2019, the HFEA received 15 requests from women to undergo MRT, of which 14 were granted.[29][30] As of 2020, if children have been born from these procedures, the details have not been published because of the wishes of the parents.[31]
Douglass Turnbull, the driving force behind mitochondrial research at Newcastle University, was awarded a knighthood in 2016.[32][33]
John Zhang team
In 2016, John Zhang and a mixed team of scientists from Mexico and New York used the spindle transfer technique to help a Jordanian woman to give birth to a baby boy. The mother had Leigh disease and already had four miscarriages and two children who had died of the disease.[34] Valery Zukin, director of the Nadiya clinic in Kyiv, Ukraine, reported in June 2018 that doctors there had used the pronuclear transfer method of MRT to help four women give birth (three boys and a girl) and three women to become pregnant (one from Sweden); the team had 14 failed attempts.[35] In January 2019 it was reported that seven babies had been born using MRT.[36] The doctors had first gotten approval from an ethical committee and a review board of the Ukrainian Association of Reproductive Medicine[37][38] and the Ukrainian Postgraduate Medical Academy, under the auspices of the Ukrainian Ministry of Healthcare;[35] there was no law in the Ukraine against MRT. One of the first children, a boy, was born to a 34-year-old woman in January 2017, and genetic test results were reported as normal.[39][40] In August and October 2017 the British HFEA authorized MRT for two women who had a genetic mutation in their mitichondria that causes myoclonic epilepsy with ragged red fibers.[41] In January 2019, Embryotools, Barcelona, Spain announced that a 32-year-old Greek woman had become pregnant using the spindle transfer technique. MRT was not legal in Spain so they had performed the trial in Greece where there was no law against MRT. They were helped by the Institute of Life in Athens, Greece and had obtained approval from the Greek National Authority of Assisted Reproduction. The pregnant Greek woman had already had four failed IVF cycles and surgery twice for endometriosis.[42]
In August 2017, in a letter to two clinics, including Zhang's, the FDA warned that the technique should not be marketed in the U.S.[43]
2018–present
In June 2018 Australian Senate's Senate Community Affairs References Committee recommended a move towards legalising MRT, and in July 2018 the Australian senate endorsed it.[44] Research and clinical applications of MRT were overseen by laws made by federal and state governments. State laws were, for the most part, consistent with federal law. In all states, legislation prohibited the use of MRT techniques in the clinic, and except for Western Australia, research on a limited range of MRT was permissible up to day 14 of embryo development, subject to a license being granted. In 2010, the Hon. Mark Butler MP, then Federal Minister for Mental Health and Ageing, had appointed an independent committee to review the two relevant acts: the Prohibition of Human Cloning for Reproduction Act 2002 and the Research Involving Human Embryos Act 2002. The committee's report, released in July 2011, recommended the existing legislation remain unchanged.[45] The Australian National Health and Medical Research Council issued two reports on legalising MRT in June 2020.[46][47] In 2022, Maeve's Law was passed by the Australian Parliament, legalising MRT under a specified mitochondrial donation licence for research and training, and in clinical settings.[48]
Singapore was also considering whether to permit the MRT in 2018.[49]
In 2018, researchers announced the use of MRT to restore function to heart tissue in cardiac-compromised newborns. The damaged heart cells absorbed mitochondria extracted from healthy tissue and returned to useful activity.[3]
Society and culture
Regulation
As of February 2016, the United States had no regulations governing mitochondrial donation, and Congress barred the FDA from evaluating any applications that involve implanting modified embryos into a woman.[50]
The United Kingdom became the first country to legalize the procedure: the UK's chief medical officer recommended it be legalized in 2013;[25] parliament passed The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations in 2015,[51][52] and the regulatory authority published regulations in 2016.[27]
Ethics
Despite the promising outcomes of the two techniques, pronuclear transfer and spindle transfer, mitochondrial gene replacement raises ethical and social concerns.[53]
Mitochondrial donation involves modification of the germline, and hence such modifications would be passed on to subsequent generations.[54] Using human embryos for in vitro research is also controversial, as embryos are created specifically for research and egg donors are induced to undergo the procedure by financial compensation.[55]
Mitochondrial donation also has the potential for psychological and emotional impacts on an offspring through an effect on the person's sense of identity. Ethicists question whether the genetic make-up of children born as a result of mitochondrial replacement might affect their emotional well-being when they become aware that they are different from other healthy children conceived from two parents.[56]
Opponents argue that scientists are "playing God" and that children with three genetic parents may suffer both psychological and physical damage.[57]
On the other hand, New York University researcher James Grifo, a critic of the American ban, has argued that society "would never have made the advances in treating infertility that we have if these bans had been imposed 10 years" earlier.[58]
On February 3, 2016, the Institute of Medicine of the National Academies of Sciences, Engineering, and Medicine issued a report, commissioned by the U.S. Food and Drug Administration, addressing whether it is ethically permissible for clinical research into mitochondrial replacement techniques (MRT) to continue. The report, titled Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations, analyzes multiple facets of the arguments surrounding MRT and concludes that it is 'ethically permissible' to continue clinical investigations of MRT, so long as certain conditions are met. It recommended that initially the technique should only be used for male embryos to ensure that DNA with potential mitochondrial disease would not be passed on.[1]
In 2018 Carl Zimmer compared the reaction to He Jiankui's human gene editing experiment to the debate over MRT.[59]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Committee on the Ethical and Social Policy Considerations of Novel Techniques for Prevention of Maternal Transmission of Mitochondrial DNA Diseases; Board on Health Sciences Policy; Institute of Medicine (2016). Claiborne, Anne; English, Rebecca; Kahn, Jeffrey. eds. Mitochondrial Replacement Techniques: Ethical, Social, and Policy Considerations. National Academies Press. ISBN 978-0-309-38870-2. https://www.nap.edu/read/21871/chapter/1. Index page with links to summaries including one page summary flyer.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Cree, L; Loi, P (January 2015). "Mitochondrial replacement: from basic research to assisted reproductive technology portfolio tool-technicalities and possible risks.". Molecular Human Reproduction 21 (1): 3–10. doi:10.1093/molehr/gau082. PMID 25425606.

- ↑ 3.0 3.1 3.2 3.3 Kolata, Gina (10 July 2018). "Dying Organs Restored to Life in Novel Experiments" (in en). The New York Times. https://www.nytimes.com/2018/07/10/health/mitochondria-transplant-heart-attack.html.
- ↑ 4.0 4.1 "33. Mitochondrial donation". Human Fertilisation and Embryology Authority. http://www.hfea.gov.uk/9931.html. Linked in HFEA announcement of the regulations issued 15 December 2016.
- ↑ Gorman, Gráinne S.; Grady, John P.; Ng, Yi; Schaefer, Andrew M.; McNally, Richard J.; Chinnery, Patrick F.; Yu-Wai-Man, Patrick; Herbert, Mary et al. (2015). "Mitochondrial Donation — How Many Women Could Benefit?". New England Journal of Medicine 372 (9): 885–887. doi:10.1056/NEJMc1500960. ISSN 0028-4793. PMID 25629662.
- ↑ Wolf, DP; Mitalipov, N; Mitalipov, S (February 2015). "Mitochondrial replacement therapy in reproductive medicine.". Trends in Molecular Medicine 21 (2): 68–76. doi:10.1016/j.molmed.2014.12.001. PMID 25573721.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 Greenfield, Andy (30 November 2016). "Scientific review of the safety and efficacy of methods to avoid mitochondrial disease through assisted conception: 2016 update". Human Fertilisation and Embryology Authority. http://www.hfea.gov.uk/docs/Fourth_scientific_review_mitochondria_2016.PDF. linked from accompanying press release
- ↑ "'Maeve's Law' to legalise mitochondrial donation through IVF passes Senate". ABC News. 31 March 2022. https://www.abc.net.au/news/science/2022-03-31/maeves-law-passes-senate-mitochondrial-donation/100954484.
- ↑ Kim Tingley for the New York Times. June 27, 2014 The Brave New World of Three-Parent I.V.F.
- ↑ Cohen, Jacques (6 April 2001). "Cytoplasmic transfer in assisted reproduction". Human Reproduction Update 7 (4): 428–435. doi:10.1093/humupd/7.4.428. PMID 11476356.
- ↑ Dale, Brian; Wilding, Martin; Botta, Giuseppe; Rasile, Marianna; Marino, Marcella; Matteo, Loredana Di; Placido, Giuseppe De; Izzo, Alfredo (2001-07-01). "Pregnancy after cytoplasmic transfer in a couple suffering from idiopathic infertility Case report" (in en). Human Reproduction 16 (7): 1469–1472. doi:10.1093/humrep/16.7.1469. ISSN 0268-1161. PMID 11425831.
- ↑ "Woman conceives through cytoplasmic transfer technology". Web India 123. 2011-11-21. http://news.webindia123.com/news/articles/India/20111121/1876130.html.
- ↑ 13.0 13.1 Kula, Shane (18 February 2016). "Three-Parent Children Are Already Here". Slate. http://www.slate.com/articles/technology/future_tense/2016/02/three_parent_babies_have_been_here_since_the_late_90s.html.
- ↑ Connor, Steve (25 August 2015). "Three-parent babies: 'As long as she's healthy, I don't care', says mother of IVF child". The Independent. https://www.independent.co.uk/news/science/three-child-babies-the-mothers-view-as-long-as-she-s-healthy-i-don-t-care-9690059.html.
- ↑ Hamzelou, Jessica (2016-09-28). "Everything you wanted to know about '3-parent' babies" (in en-US). The New Scientist. https://www.newscientist.com/article/2107451-everything-you-wanted-to-know-about-3-parent-babies/.
- ↑ Chen, Serena H.; Pascale, Claudia; Jackson, Maria; Szvetecz, Mary Ann; Cohen, Jacques (December 2016). "A limited survey-based uncontrolled follow-up study of children born after ooplasmic transplantation in a single centre". Reproductive BioMedicine Online 33 (6): 737–744. doi:10.1016/j.rbmo.2016.10.003. PMID 27789184.
- ↑ Alleyne, Richard (12 November 2009). "'Three parent babies' take a step closer to reality". Telegraph. https://www.telegraph.co.uk/news/health/news/6546448/Three-parent-babies-take-a-step-closer-to-reality.html.
- ↑ Naik, Gautam (27 November 2012). "DNA Switch Shows Promise Against Genetic Disease". Wall Street Journal. https://www.wsj.com/articles/SB10001424052970204076204578076530392342640.
- ↑ Fogleman, Sarah (2016-09-20). "CRISPR/Cas9 and mitochondrial gene replacement therapy: promising techniques and ethical considerations" (in en-US). The American Journal of Stem Cells 5 (2): 39–52. PMID 27725916.
- ↑ Donaldson, Liam (16 August 2000). "Stem cell research: medical progress with responsibility". UK Department of Health. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4065085.pdf.
- ↑ Randerson, James (19 October 2004). "Scientists seek to create 'three-parent' babies". New Scientist. https://www.newscientist.com/article/dn6547-scientists-seek-to-create-three-parent-babies/.
- ↑ Boseley, Sarah (2010-04-14). "Scientists reveal gene-swapping technique to thwart inherited diseases". London: Guardian. https://www.theguardian.com/science/2010/apr/14/scientists-gene-swap-technique-disease.
- ↑ Craven, Lyndsey; Tuppen, Helen A.; Greggains, Gareth D.; Harbottle, Stephen J.; Murphy, Julie L.; Cree, Lynsey M.; Murdoch, Alison P.; Chinnery, Patrick F. et al. (2010). "Pronuclear transfer in human embryos to prevent transmission of mitochondrial DNA disease". Nature 465 (7294): 82–85. doi:10.1038/nature08958. PMID 20393463. Bibcode: 2010Natur.465...82C.

- ↑ Greenfield, Andy, ed (June 2014). Third scientific review of the safety and efficacy of methods to avoid mitochondrial disease through assisted conception: 2014 update. UK: Human Fertilisation and Embryology Authority. http://www.hfea.gov.uk/docs/Third_Mitochondrial_replacement_scientific_review.pdf. Retrieved 2 March 2016.
- ↑ 25.0 25.1 Connor, Steve (28 June 2013). "UK becomes first country in world to approve IVF using genes of three". The Independent. https://www.independent.co.uk/news/science/uk-becomes-first-country-in-world-to-approve-ivf-using-genes-of-three-parents-8677595.html.
- ↑ Craven, Lindsay (29 September 2015). "Research into Policy: A Brief History of Mitochondrial Donation". Stem Cells 34 (2): 265–267. doi:10.1002/stem.2221. PMID 26418557.
- ↑ 27.0 27.1 27.2 Gallagher, James (2016-12-15). "Babies made from three people approved in UK" (in en-GB). BBC News. https://www.bbc.co.uk/news/health-38328097.
- ↑ Gallagher, James (2017-03-16). "Three-person baby licence granted" (in en-GB). BBC News. https://www.bbc.co.uk/news/health-39292381.
- ↑ "UK doctors select first women to have 'three-person babies'" (in en-GB). The Guardian. 2018-02-02. https://www.theguardian.com/science/2018/feb/01/permission-given-to-create-britains-first-three-person-babies.
- ↑ Doyle-Price, Jackie (2019-04-05). "Embryos - Question for Department of Health and Social Care" (in en). https://questions-statements.parliament.uk/written-questions/detail/2019-04-05/241389.
- ↑ Elliott, Tim (2020-03-20). "How far should genetic engineering go to allow this couple to have a healthy baby?" (in en). https://www.smh.com.au/national/how-far-should-genetic-engineering-go-to-allow-this-couple-to-have-a-healthy-baby-20191231-p53nx2.html.
- ↑ No. 61608. 2016-06-11. p. B2. https://www.thegazette.co.uk/London/issue/61608/supplement/B2
- ↑ "Birthday honours: Mitochondrial disease doctor recognised". 2016-06-10. https://www.bbc.co.uk/news/health-36499807.
- ↑ Roberts, Michelle (2016-09-27). "First 'three person baby' born using new method" (in en-GB). BBC News. https://www.bbc.co.uk/news/health-37485263.
- ↑ 35.0 35.1 Stein, Rob (2018-06-06). "Clinic Claims Success In Making Babies With 3 Parents' DNA" (in en). National Public Radio. https://www.npr.org/sections/health-shots/2018/06/06/615909572/inside-the-ukrainian-clinic-making-3-parent-babies-for-women-who-are-infertile?t=1531299279185.
- ↑ Walas, Dorothy (2019-01-05). "Three parent baby in Ukraine" (in en-GB). https://www.eggdonationfriends.com/3-parent-baby-groundbreaking-pronucleus-transfer-interview-with-nadiya-clinic/.
- ↑ Reardon, Sara (20 October 2016). "Reports of 'three-parent babies' multiply". Nature News. doi:10.1038/nature.2016.20849. http://www.nature.com/news/reports-of-three-parent-babies-multiply-1.20849.
- ↑ Coghlan, Andy (2016-10-10). "Exclusive: '3-parent' baby method already used for infertility" (in en-US). New Scientist. https://www.newscientist.com/article/2108549-exclusive-3-parent-baby-method-already-used-for-infertility/.
- ↑ Moody, Oliver (2017-01-18). "Three-parent baby born to 'infertile' woman using controversial new IVF" (in en). The Times: p. 12. http://www.thetimes.co.uk/article/three-parent-baby-born-to-infertile-woman-using-controversial-new-ivf-l5zhpx0qz.
- ↑ Stein, Rob (2018-06-06). "Her Son Is One Of The Few Children To Have 3 Parents' DNA" (in en). National Public Radio. https://www.npr.org/sections/health-shots/2018/06/06/616334508/her-son-is-one-of-the-few-children-to-have-3-parents.
- ↑ Hamzelou, Jessica (2018-02-02). "First UK three-parent babies could be born this year" (in en-US). New Scientist. https://www.newscientist.com/article/2160120-first-uk-three-parent-babies-could-be-born-this-year/.
- ↑ Willows, Jennifer (2019-01-29). "Birth expected in mitochondrial donation for infertility trial - BioNews". https://www.bionews.org.uk/page_141058.
- ↑ "'Three parent' technique must not be marketed in US, says FDA". New Scientist. 9 August 2017. https://www.newscientist.com/article/mg23531384-100-three-parent-technique-must-not-be-marketed-in-us-says-fda/.
- ↑ Pritchard, Sarah (2018-07-02). "Australian Senate endorses mitochondrial donation - BioNews" (in en). https://www.bionews.org.uk/page_136808.
- ↑ "Mitochondrial Donation - Mito Foundation (formerly AMDF)" (in en-US). Mito Foundation (formerly AMDF). https://www.mito.org.au/mitochondrial-donation/.
- ↑ "Australian Government CEO Statement: Should Australia introduce mitochondrial donation?". 5 June 2020. https://www.nhmrc.gov.au/sites/default/files/documents/attachments/CEO-statement-mitochondrial.pdf.
- ↑ "Mitochondrial Donation". November 2019. https://www.nhmrc.gov.au/health-advice/all-topics/mitochondrial-donation.
- ↑ corporateName=Commonwealth Parliament; address=Parliament House, Canberra. "Mitochondrial Donation Law Reform (Maeve's Law) Bill 2021" (in en-AU). https://www.aph.gov.au/Parliamentary_Business/Bills_LEGislation/Bills_Search_Results/Result?bId=r6697.
- ↑ Ong, Sandy (2018-06-06). "Singapore could become the second country to legalize mitochondrial replacement therapy" (in en). Science; American Association for the Advancement of Science. https://www.science.org/content/article/singapore-could-become-second-country-legalize-mitochondrial-replacement-therapy.
- ↑ Oswald, Kirtsy (8 February 2016). "Panel recommends FDA approval of mitochondrial donation". Bio News. http://www.bionews.org.uk/page_614550.asp.
- ↑ Gallagher, James (24 February 2015). "UK approves three-person babies". BBC News. https://www.bbc.com/news/health-31594856.
- ↑ The Human Fertilisation and Embryology (Mitochondrial Donation) Regulations 2015 No. 572
- ↑ Baylis, F (June 2013). "The ethics of creating children with three genetic parents.". Reproductive Biomedicine Online 26 (6): 531–4. doi:10.1016/j.rbmo.2013.03.006. PMID 23608245.
- ↑ Darnovsky M (2013). "A slippery slope to human germline modification". Nature 499 (7457): 127. doi:10.1038/499127a. PMID 23846625. Bibcode: 2013Natur.499..127D.
- ↑ Amato P.; Tachibana M.; Sparman M.; Mitalipov S. (2014). "Three-parent in vitro fertilization: Gene replacement for the prevention of inherited mitochondrial diseases". Fertility and Sterility 101 (1): 31–35. doi:10.1016/j.fertnstert.2013.11.030. PMID 24382342.
- ↑ "CGS : 3-Person IVF: A Resource Page". Center for Genetics and Society. December 19, 2016. http://www.geneticsandsociety.org/article.php?id=6527#1a.
- ↑ Check E (November 2005). "Gene study raises fears for three-parent babies". Nature 438 (7064): 12. doi:10.1038/438012a. PMID 16267521. Bibcode: 2005Natur.438...12C.
- ↑ Maxine, Frith (2003-10-14). "Ban on scientists trying to create three-parent baby". The Independent (UK).
- ↑ Zimmer, Carl (1 December 2018). "Genetically Modified People Are Walking Among Us - And, so far, they're just fine. America needs a sober debate about the pros and cons of Crispr instead of a paranoid ban on the technology.". The New York Times. https://www.nytimes.com/2018/12/01/sunday-review/crispr-china-babies-gene-editing.html.
 |

