Physics:Electrochromism

Electrochromism is a phenomenon in which a material displays changes in color or opacity in response to an electrical stimulus.[2] In this way, a smart window made of an electrochromic material can block specific wavelengths of ultraviolet, visible or (near) infrared light. The ability to control the transmittance of near-infrared light can increase the energy efficiency of a building, reducing the amount of energy needed to cool during summer and heat during winter.[1][3]
As the color change is persistent and energy needs only to be applied to effect a change, electrochromic materials are used to control the amount of light and heat allowed to pass through a surface, most commonly "smart windows". One popular application is in the automobile industry where it is used to automatically tint rear-view mirrors in various lighting conditions.
Principle
The phenomenon of electrochromism occurs in some transition metal oxides which conduct both electrons and ions, such as tungsten trioxide (WO3).[4] These oxides have octahedral structures of oxygen which surround a central metal atom and are joined together at the corners. This arrangement produces a three-dimensional nanoporous structure with "tunnels" between individual octahedral segments. These tunnels allow dissociated ions to pass through the substance when they are motivated by an electric field. Common ions used for this purpose are H+ and Li+.[5] [6]
The electric field is typically induced by two flat, transparent electrodes which sandwich the ion-containing layers. As a voltage is applied across these electrodes, the difference in charge between the two sides causes the ions to penetrate the oxide as the charge-balancing electrons flow between the electrodes. These electrons change the valency of the metal atoms in the oxide, reducing their charge, as in the following example of tungsten trioxide:[7]
- WO3 + n(H+ + e−) → HnWO3
This is a redox reaction since the electroactive metal accepts electrons from the electrodes, forming a half-cell.[7] Strictly speaking, the electrode as a chemical unit comprises the flat plate as well as the semiconducting substance in contact with it. However, the term "electrode" often refers to only the flat plate(s), more specifically called the electrode "substrate".[8]
Photons that reach the oxide layer can cause an electron to move between two nearby metal ions. The energy provided by the photon causes the movement of an electron which in turn causes optical absorption of the photon.[9] For example, the following process occurs in tungsten oxide for two tungsten ions a and b:[10]
- W5+a + W6+b + photon → W6+a + W5+b
Electrochromic materials
Electrochromic materials, also known as chromophores, affect the optical color or opacity of a surface when a voltage is applied.[7][11] Among the metal oxides, tungsten oxide (WO3) is the most extensively studied and well-known electrochromic material.[12] Others include molybdenum,[13] titanium[14] and niobium oxides,[15] although these are less effective optically.
Viologens are a class of organic materials[16][17] that are being intensively investigated for electrochromic applications.[18] These 4,4′-bipyridine compounds display reversible color changes between a colorless and a deep-blue color due to redox reactions. Researchers can "tune" them to a deep blue or intense green.[11]
As organic materials, viologens are seen as promising alternatives for electronic applications, compared to metal-based systems, which tend to be expensive, toxic, and a problem to recycle.[16] Possible advantages of viologens include their optical contrast, coloration efficiency, redox stability, ease of design, and potential to scale up for large-area preparation.[18]
Viologens have been used with phenlyenediamine by Gentex Corporation, which has commercialized auto-dimming rearview mirrors[18] and smart windows in Boeing 787 aircraft.[11] Viologen has been used in conjunction with titanium dioxide (TiO2, also known as titania) in the creation of small digital displays.[19][20] A variety of conducting polymers are also of interest for displays, including polypyrrole, PEDOT, and polyaniline.[21]
Synthesis of tungsten oxide
Many methods have been used to synthesize tungsten oxide, including chemical vapor deposition (CVD), sputtering, thermal evaporation, spray pyrolysis (from a vapor or sol-gel), and hydrothermal synthesis (from a liquid).[22] In industry, sputtering is the most common method for the deposition of tungsten oxide. For material synthesis, sol-gel process is widely used due to its advantages of simple process, low cost, and easy control.[23]
Sol-gel process
In the sol-gel process of tungsten trioxide, WCl6 is dissolved in alcohol and then oxidized by purging O2 into its solution:
- 2WCl6 + 3O2 → 3WO3 + 6Cl2
The formation of H2 is performed by the reaction of alcohol and chlorine that used for the reduction of WO3 to obtain a blue solution of HWO3:
- (CH3)2CH–OH + 3Cl2 → (Cl3C)2=O + 4H2
- 2WO3 + H2 → 2HWO3
WO3 nanoparticles can also be obtained by precipitation of ammonium tungstate para pentahydrate, (NH4)10W12O41⋅5H2O, or nitric acid, HNO3, under acidic conditions from aqueous solutions.[24]
Working principle of electrochromic windows
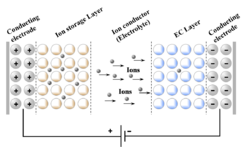
Multiple layers are needed for a functional smart window with electrochromic characteristics.[3] The first and last are transparent glass made of silica (SiO2), the two electrodes are needed to apply the voltage, which in turn will push (or pull) Li+ ions from the ion storage layer, through the electrolyte into the electrochromic material (or vice versa). Applying a high voltage (4 V or more) will push lithium-ions into the electrochromic layer, deactivating the electrochromic material. The window is fully transparent now. By applying a lower voltage (2.5 V for example) the concentration of Li-ions in the electrochromic layer decreases, thus activating (N)IR-active tungsten oxide.[25][3] This activation causes reflection of infrared light, thus lowering the greenhouse effect, which in turn reduces the amount of energy needed for air conditioning.[26] Depending on the electrochromic material used, different parts of the spectrum can be blocked, this way UV, visible and IR light can be independently reflected at the will of a user.[3]
Applications

Several electrochromic devices have been developed. Electrochromism is commonly used in the production of electrochromic windows or "smart glass",[3][1] and more recently electrochromic displays on paper substrate as anti-counterfeiting systems integrated into packaging.[27] NiO materials have been widely studied as counter electrodes for complementary electrochromic devices, particularly for smart windows.[28][29]

ICE 3 high speed trains use electrochromic glass panels between the passenger compartment and the driver's cabin. The standard mode is clear, and can be switched by the driver to frosted.[30] Electrochromic windows are used in the Boeing 787 Dreamliner, allowing crew and passengers to control the transparency of the windows and prevent glare.[31]
See also
Further reading
- Granqvist, C.G. (2002). Handbook of Inorganic Electrochromic Materials. Elsevier. ISBN 978-0-08-053290-5. https://books.google.com/books?id=MYd1Np3yO-8C&pg=PP1.
- Lin, Feng; Nordlund, Dennis; Weng, Tsu-Chien et al. (2013). "Origin of Electrochromism in High-Performing Nanocomposite Nickel Oxide". ACS Applied Materials & Interfaces (American Chemical Society) 5 (9): 3643–3649. doi:10.1021/am400105y. PMID 23547738.
- Moulki, Hakim; Park, Dae Hoon; Min, Bong-Ki et al. (15 July 2012). "Improved electrochromic performances of NiO based thin films by lithium addition: From single layers to devices". Electrochimica Acta 74: 46–52. doi:10.1016/j.electacta.2012.03.123.
- Lin, Feng; Cheng, Jifang; Engtrakul, Chaiwat et al. (2012). "In situ crystallization of high performing WO3-based electrochromic materials and the importance for durability and switching kinetics". Journal of Materials Chemistry 22 (33): 16817–16823. doi:10.1039/c2jm32742b.
- Deb, S. K. (1969). "A Novel Electrophotographic System". Applied Optics 8 (S1): 192–195. doi:10.1364/AO.8.S1.000192. PMID 20076124. Bibcode: 1969ApOpt...8S.192D.
- Deb, S. K. (1973). "Optical and photoelectric properties and colour centres in thin films of tungsten oxide". Philosophical Magazine 27 (4): 801–822. doi:10.1080/14786437308227562. Bibcode: 1973PMag...27..801D.
- Gillaspie, Dane T.; Tenent, Robert C.; Dillon, Anne C. (2010). "Metal-oxide films for electrochromic applications: present technology and future directions". Journal of Materials Chemistry 20 (43): 9585–9592. doi:10.1039/C0JM00604A.
- Danine, A.; Cojocaru, L.; Faure, C. et al. (20 May 2014). "Room Temperature UV treated WO3 thin films for electrochromic devices on paper substrate". Electrochimica Acta 129: 113–119. doi:10.1016/j.electacta.2014.02.028.
- ; Faure, Cyril & Campet, Guy et al."Electrochromic device comprising three or four layers" WO patent 2014135804, issued September 12, 2014
References
- ↑ 1.0 1.1 1.2 Mortimer, R.J. (2011). "Electrochromic Materials". Annu. Rev. Mater. Res.. 41. pp. 241–268. doi:10.1146/annurev-matsci-062910-100344. Bibcode: 2011AnRMS..41..241M.
- ↑ Chua, Ming Hui; Tang, Tao; Ong, Kok Haw; Neo, Wei Teng; Xu, Jian Wei (2019). "Chapter 1 Introduction to Electrochromism" (in en). Electrochromic Smart Materials: Fabrication and Applications. Smart Materials Series. Royal Society of Chemistry. pp. 1–21. doi:10.1039/9781788016667-00001. ISBN 978-1-78801-143-3. https://pubs.rsc.org/en/content/chapterhtml/2019/bk9781788011433-00001?isbn=978-1-78801-143-3&sercode=bk. Retrieved 29 July 2022.
- ↑ 3.0 3.1 3.2 3.3 3.4 Miller, Brittney J. (8 June 2022). "How smart windows save energy". Knowable Magazine. doi:10.1146/knowable-060822-3. https://knowablemagazine.org/article/technology/2022/how-smart-windows-save-energy. Retrieved 15 July 2022.
- ↑ Somani, Prakash R.; Radhakrishnan, S. (26 September 2001). "Electrochromic materials and devices: present and future". Materials Chemistry and Physics (Elsevier) 77: 117–133. doi:10.1016/S0254-0584(01)00575-2. http://nathan.instras.com/documentDB/paper-134.pdf. Retrieved 22 August 2019.
- ↑ Granqvist, C.G. (2015). Eco-efficient Materials for Mitigating Building Cooling Needs. Elsevier. pp. 460–464. ISBN 978-1-78242-380-5.
- ↑ Brus, Jiri; Czernek, Jiri; Urbanova, Martina; Rohlíček, Jan; Plecháček, Tomáš (2020). "Transferring Lithium Ions in the Nanochannels of Flexible Metal–Organic Frameworks Featuring Superchaotropic Metallacarborane Guests: Mechanism of Ionic Conductivity at Atomic Resolution". ACS Appl. Mater. Interfaces 12 (42): 47447–47456. doi:10.1021/acsami.0c12293. PMID 32975402.
- ↑ 7.0 7.1 7.2 Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. (2007). Electrochromism and Electrochromic Devices. Cambridge University Press. ISBN 978-0-521-82269-5.
- ↑ (in en) NASA Reference Publication. National Aeronautics and Space Administration, Scientific and Technical Information Branch. 1979. pp. x. https://books.google.com/books?id=-lgjAAAAMAAJ&pg=PA13. Retrieved 29 July 2022.
- ↑ "Colours of Transition Metal Ions in Aqueous Solution". 5 March 2014. https://www.compoundchem.com/2014/03/05/colours-of-transition-metal-ions-in-aqueous-solution/.
- ↑ Deepa, M.; Joshi, A. G.; Srivastava, A. K.; Shivaprasad, S. M.; Agnihotry, S. A. (2006). "Electrochromic Nanostructured Tungsten Oxide Films by Sol-gel: Structure and Intercalation Properties". Journal of the Electrochemical Society 153 (5): C365. doi:10.1149/1.2184072. Bibcode: 2006JElS..153C.365D.
- ↑ 11.0 11.1 11.2 Wang, Yang; Runnerstrom, Evan L.; Milliron, Delia J. (7 June 2016). "Switchable Materials for Smart Windows" (in en). Annual Review of Chemical and Biomolecular Engineering 7 (1): 283–304. doi:10.1146/annurev-chembioeng-080615-034647. ISSN 1947-5438. PMID 27023660. https://www.annualreviews.org/doi/pdf/10.1146/annurev-chembioeng-080615-034647. Retrieved 29 July 2022.
- ↑ Can, Fabien; Courtois, Xavier; Duprez, Daniel (2 June 2021). "Tungsten-Based Catalysts for Environmental Applications". Catalysts 11 (6): 703. doi:10.3390/catal11060703. https://mdpi-res.com/d_attachment/catalysts/catalysts-11-00703/article_deploy/catalysts-11-00703-v2.pdf?version=1623117112. Retrieved 29 July 2022.
- ↑ Jinmin, Wang; Lijun, H. O. U.; Dongyun, M. A. (20 May 2021). "Molybdenum Oxide Electrochromic Materials and Devices" (in en). Journal of Inorganic Materials 36 (5): 461. doi:10.15541/jim20200416. ISSN 1000-324X.
- ↑ Eyovge, Cavit; Deenen, Cristian S.; Ruiz-Zepeda, Francisco; Bartling, Stephan; Smirnov, Yury; Morales-Masis, Monica; Susarrey-Arce, Arturo; Gardeniers, Han (27 August 2021). "Color Tuning of Electrochromic TiO 2 Nanofibrous Layers Loaded with Metal and Metal Oxide Nanoparticles for Smart Colored Windows" (in en). ACS Applied Nano Materials 4 (8): 8600–8610. doi:10.1021/acsanm.1c02231. ISSN 2574-0970. PMID 34485847. PMC 8406417. https://doi.org/10.1021/acsanm.1c02231.
- ↑ Ong, Gary K.; Saez Cabezas, Camila A.; Dominguez, Manuel N.; Skjærvø, Susanne Linn; Heo, Sungyeon; Milliron, Delia J. (14 January 2020). "Electrochromic Niobium Oxide Nanorods" (in en). Chemistry of Materials 32 (1): 468–475. doi:10.1021/acs.chemmater.9b04061. ISSN 0897-4756. https://doi.org/10.1021/acs.chemmater.9b04061.
- ↑ 16.0 16.1 Striepe, Laura; Baumgartner, Thomas (1 December 2017). "Viologens and Their Application as Functional Materials" (in en). Chemistry - A European Journal 23 (67): 16924–16940. doi:10.1002/chem.201703348. PMID 28815887. https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/chem.201703348. Retrieved 29 July 2022.
- ↑ Kathiresan, Murugavel; Ambrose, Bebin; Angulakshmi, Natarajan; Mathew, Deepa Elizabeth; Sujatha, Dhavamani; Stephan, Arul Manuel (2021). "Viologens: a versatile organic molecule for energy storage applications" (in en). Journal of Materials Chemistry A 9 (48): 27215–27233. doi:10.1039/D1TA07201C. ISSN 2050-7488. https://doi.org/10.1039/D1TA07201C. Retrieved 29 July 2022.
- ↑ 18.0 18.1 18.2 Shah, Kwok Wei; Wang, Su-Xi; Soo, Debbie Xiang Yun; Xu, Jianwei (8 November 2019). "Viologen-Based Electrochromic Materials: From Small Molecules, Polymers and Composites to Their Applications". Polymers 11 (11): 1839. doi:10.3390/polym11111839. PMID 31717323.
- ↑ De Matteis, Valeria; Cannavale, Alessandro; Ayr, Ubaldo (13 December 2020). "Titanium Dioxide in Chromogenic Devices: Synthesis, Toxicological Issues, and Fabrication Methods". Applied Sciences 10 (24): 8896. doi:10.3390/app10248896. https://mdpi-res.com/d_attachment/applsci/applsci-10-08896/article_deploy/applsci-10-08896.pdf?version=1607851327. Retrieved 29 July 2022.
- ↑ Zheng, Yanxing; Wang, Jiwei; Tang, Xinqiao; Zhang, Lei; Meng, Fanbao (1 March 2020). "Liquid-crystalline behavior and ferroelectric property of viologen-based ionic liquid crystals" (in en). Journal of Molecular Liquids 301: 112369. doi:10.1016/j.molliq.2019.112369. ISSN 0167-7322. https://www.sciencedirect.com/science/article/abs/pii/S0167732219339893.
- ↑ Ouyang, Jianyong (September 2021). "Application of intrinsically conducting polymers in flexible electronics" (in en). SmartMat 2 (3): 263–285. doi:10.1002/smm2.1059. ISSN 2688-819X. https://onlinelibrary.wiley.com/doi/full/10.1002/smm2.1059.
- ↑ Zheng, Haidong; Ou, Jian Zhen; Strano, Michael S.; Kaner, Richard B.; Mitchell, Arnan; Kalantar-zadeh, Kourosh (2011-05-24). "Nanostructured Tungsten Oxide – Properties, Synthesis, and Applications". Advanced Functional Materials 21 (12): 2175–2196. doi:10.1002/adfm.201002477. ISSN 1616-301X.
- ↑ Lai, Wei Hao; Su, Yen Hsun; Teoh, Lay Gaik; Tsai, Yuan Tsung; Hon, Min Hsiung (2007). "Synthesis of Tungsten Oxide Particles by Chemical Deposition Method". Materials Transactions 48 (6): 1575–1577. doi:10.2320/matertrans.mep2007057. ISSN 1345-9678.
- ↑ Supothina, Sitthisuntorn; Seeharaj, Panpailin; Yoriya, Sorachon; Sriyudthsak, Mana (August 2007). "Synthesis of tungsten oxide nanoparticles by acid precipitation method". Ceramics International 33 (6): 931–936. doi:10.1016/j.ceramint.2006.02.007. ISSN 0272-8842.
- ↑ Woodford, Chris (April 23, 2021). "How do electrochromic (smart glass) windows work?". Explain that Stuff. https://www.explainthatstuff.com/electrochromic-windows.html.
- ↑ "Smart windows that protect against solar radiation can help reduce greenhouse gases" (in en). Tech Xplore. September 1, 2021. https://techxplore.com/news/2021-09-smart-windows-solar-greenhouse-gases.html.
- ↑ Glogic, Edis; Futsch, Romain; Thenot, Victor; Iglesias, Antoine; Joyard-Pitiot, Blandine; Depres, Gael; Rougier, Aline; Sonnemann, Guido (6 September 2021). "Development of Eco-Efficient Smart Electronics for Anticounterfeiting and Shock Detection Based on Printable Inks" (in en). ACS Sustainable Chemistry & Engineering 9 (35): 11691–11704. doi:10.1021/acssuschemeng.1c02348. ISSN 2168-0485. https://pubs.acs.org/doi/abs/10.1021/acssuschemeng.1c02348.
- ↑ Islam, Shakirul M.; Hernandez, Tyler S.; McGehee, Michael D.; Barile, Christopher J. (March 2019). "Hybrid dynamic windows using reversible metal electrodeposition and ion insertion". Nature Energy 4 (3): 223–229. doi:10.1038/s41560-019-0332-3. Bibcode: 2019NatEn...4..223I. https://www.colorado.edu/lab/mcgehee/sites/default/files/attached-files/nature_energy_paper.pdf.
- ↑ Chen, Po-Wen; Chang, Chen-Te; Ko, Tien-Fu; Hsu, Sheng-Chuan; Li, Ke-Ding; Wu, Jin-Yu (21 May 2020). "Fast response of complementary electrochromic device based on WO3/NiO electrodes" (in en). Scientific Reports 10 (1): 8430. doi:10.1038/s41598-020-65191-x. ISSN 2045-2322. PMID 32439890. PMC 7242463. Bibcode: 2020NatSR..10.8430C. https://doi.org/10.1038/s41598-020-65191-x. Retrieved 29 July 2022.
- ↑ "Introducing German Railways InterCity Express trains". https://www.seat61.com/trains-and-routes/ice.htm.
- ↑ Hardiman, Jake (17 June 2021). "Why The Boeing 787 Has Dimmable Windows". Simple Flying. https://simpleflying.com/boeing-787-dimmable-windows-why/.
External links
- Tutorial on electrochromatic displays at Gent University (archived from the original on 6 January 2012)
- Article on energy efficiency of electrochromic windows at National Renewable Energy Laboratory (archived from the original on 21 July 2017)
- Video of electrochromic glass changing from translucent to transparent at YouTube
 |
