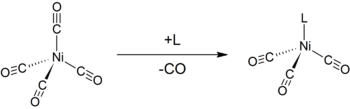Physics:Tolman electronic parameter

The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v symmetric complex, [LNi(CO)3] by infrared spectroscopy, where L is the ligand of interest. [LNi(CO)3] was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0).[1][2] The shift in ν(CO) is used to infer the electronic properties of a ligand, which can aid in understanding its behavior in other complexes. The analysis was introduced by Chadwick A. Tolman.
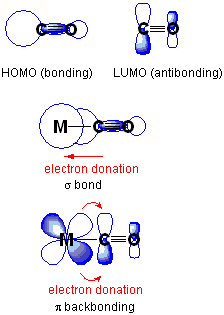
The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by π backbonding: the metal forms a π bond with the carbonyl ligand by donating electrons through its d orbitals into the empty π* anti-bonding orbitals on CO. This interaction strengthens the metal-carbon bond but also weakens the carbon-oxygen bond, resulting in a lower vibrational frequency. If other ligands increase the density of π electrons on the metal, the C-O bond is weakened and ν(CO) decreases further; conversely, if other ligands compete with CO for π backbonding, ν(CO) increases.
| L | ν(CO) cm−1 |
|---|---|
| P(t-Bu)3 | 2056.1 |
| P(NMe2)3 | 2061.9 |
| PMe3 | 2064.1 |
| P(C6H4OMe)3 | 2066 |
| PPh3 | 2068.9 |
| P(C6H4F)3 | 2071.3 |
| P(OEt)3 | 2076.3 |
| PCl3 | 2097.0 |
| PF3 | 2110.8 |
Other ligand electronic parameters
Several other scales have been proposed for the ranking of the donor properties of ligands. The HEP scale ranks ligands on the basis of the 13C NMR shift of a reference ligand.[3] Lever's electronic parameter ranking is related to the Ru(II/III) couple.[4] Another scale evaluated ligands on the basis of the redox couples of [Cr(CO)5L]0/+.[5]
In a treatment akin to the TEP analysis, the donor properties of N-heterocyclic carbene (NHC) ligands have been ranked according to IR data recorded on cis-[RhCl(NHC)(CO)2] complexes.[6][7]
See also
- Metal carbonyl
- Tolman cone angle
References
- ↑ Robert H. Crabtree (2005). "Carbonyls, Phosphine Complexes, and Ligand Substitution Reactions". The Organometallic Chemistry of the Transition Metals. pp. 87–124. doi:10.1002/0471718769.ch4. ISBN 9780471718765.
- ↑ 2.0 2.1 Tolman, C. A. (1977). "Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis". Chem. Rev. 77 (3): 313–348. doi:10.1021/cr60307a002.
- ↑ Teng, Qiaoqiao; Huynh, Han Vinh (2017). "A Unified Ligand Electronic Parameter Based on C NMR Spectroscopy of N-Heterocyclic Carbene Complexes". Dalton Transactions 46 (3): 614–627. doi:10.1039/C6DT04222H. PMID 27924321.
- ↑ Lever, A. B. P. (1990). "Electrochemical parametrization of metal complex redox potentials, using the ruthenium(III)/Ruthenium(II) couple to generate a ligand electrochemical series". Inorganic Chemistry 29 (6): 1271–1285. doi:10.1021/ic00331a030.
- ↑ Chatt, Joseph; Kan, C. T.; Leigh, G. Jeffery; Pickett, Christopher J.; Stanley, David R. (1980). "Transition-metal binding sites and ligand parameters". Journal of the Chemical Society, Dalton Transactions (10): 2032. doi:10.1039/DT9800002032.
- ↑ Nonnenmacher, Michael; Buck, Dominik M; Kunz, Doris (23 August 2016). "Experimental and theoretical investigations on the high-electron donor character of pyrido-annelated N-heterocyclic carbenes". Beilstein Journal of Organic Chemistry 12: 1884–1896. doi:10.3762/bjoc.12.178. PMID 27829895.
- ↑ Huynh, Han Vinh (30 March 2018). "Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination". Chemical Reviews 118 (19): 9457–9492. doi:10.1021/acs.chemrev.8b00067. PMID 29601194.
Further reading
- Tonner, Ralf; Frenking, Gernot (2009). "Tolman's Electronic Parameters for Divalent Carbon(0) Compounds". Organometallics 28 (13): 3901–3905. doi:10.1021/om900206w.
- Gusev, Dmitry G. (2009). "Electronic and Steric Parameters of 76 N-Heterocyclic Carbenes in Ni(CO)3(NHC)". Organometallics 28 (22): 6458–6461. doi:10.1021/om900654g.
 |