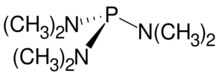
Chemistry:Tris(dimethylamino)phosphine
| |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N′,N′,N′′,N′′-Hexamethylphosphanetriamine | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
| 906778 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H18N3P | |
| Molar mass | 163.205 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.898 g/cm3 |
| Boiling point | 49 °C (120 °F; 322 K) 11 torr |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H226 | |
| P210, P233, P240, P241, P242, P243, P280, P303+361+353, P370+378, P403+235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tris(dimethylamino)phosphine is an organophosphorus compound with the formula P(NMe2)3 (Me = methyl). It is a colorless oil at room temperature, and is one of the most common aminophosphines. Its structure has been determined by X-ray crystallography.[2]
Tris(dimethylamino)phosphine acts as a base. It reacts with oxygen to give hexamethylphosphoramide, O=P(NMe2)3, and with sulfur to give the corresponding compound hexamethylthiophosphoramide, S=P(NMe2)3. It can also act as a ligand, forming complexes with a variety of metal centers.[3] Its steric and electronic properties are similar to those of triisopropylphosphine.[4]
Because of its affinity for sulfur, tris(dimethylamino)phosphine is also effective as a desulfurization agent, e.g., in the conversion of dibenzyl disulfide into dibenzyl sulfide:[5]
- PhCH2SSCH2Ph + P(NMe2)3 → S=P(NMe2)3 + PhCH2SCH2Ph (Ph = phenyl)
References
- ↑ 1.0 1.1 This name is also used to refer to hexamethylphosphoramide
- ↑ Mitzel, Norbert W.; Smart, Bruce A.; Dreihäupl, Karl-Heinz; Rankin, David W. H.; Schmidbaur, Hubert (1996). "Low Symmetry in P(NR2)3 Skeletons and Related Fragments: An Inherent Phenomenon". Journal of the American Chemical Society 118 (50): 12673–12682. doi:10.1021/ja9621861.
- ↑ King, R. B. (1963). "Complexes of Trivalent Phosphorus Derivatives. II. Metal Carbonyl Complexes of Tris(dimethylamino)-phosphine". Inorganic Chemistry 2 (5): 936–944. doi:10.1021/ic50009a014.
- ↑ Tolman, C. A. (1977). "Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis". Chem. Rev. 77 (3): 313–348. doi:10.1021/cr60307a002.
- ↑ Harpp, David N.; Smith, Roger A. (1978). "Sulfide Synthesis: Benzyl Sulfide". Org. Synth. 58: 138. doi:10.15227/orgsyn.058.0138.
 |
