Shapiro Senapathy algorithm
The Shapiro Senapathy algorithm (S&S) is an algorithm for predicting splice junctions in genes of animals and plants.[1][2] This algorithm has been used to discover disease-causing splice site mutations and cryptic splice sites.
The algorithm
A splice site is the border between an exon and intron in a gene. These sites contain a particular sequence motif, which is necessary for recognition and processing by the RNA splicing machinery.[1]
The S&S algorithm uses sliding windows of eight nucleotides, corresponding to the length of the splice site sequence motif, to identify these conserved sequences and thus potential splice sites.[1] Using a weighted table of nucleotide frequencies, the S&S algorithm outputs a consensus-based percentage for the possibility of the window containing a splice site.[1]
The S&S algorithm serves as the basis of other software tools, such as Human Splicing Finder,[3] Splice-site Analyzer Tool,[4] dbass (Ensembl),[5] Alamut,[6] and SROOGLE.[7]
Cancer gene discovery using S&S
By using the S&S algorithm, mutations and genes that cause many different forms of cancer have been discovered. For example, genes causing commonly occurring cancers including breast cancer,[8][9][10] ovarian cancer,[11][12][13] colorectal cancer,[14][15][16] leukemia,[17][18] head and neck cancers,[19][20] prostate cancer,[21][22] retinoblastoma,[23][24] squamous cell carcinoma,[25][26][27] gastrointestinal cancer,[28][29] melanoma,[30][31] liver cancer,[32][33] Lynch syndrome,[34][35][15] skin cancer,[25][36][37] and neurofibromatosis[38][39] have been found. In addition, splicing mutations in genes causing less commonly known cancers including gastric cancer,[40][41][28] gangliogliomas,[42][43] Li-Fraumeni syndrome, Loeys–Dietz syndrome, Osteochondromas (bone tumor), Nevoid basal cell carcinoma syndrome,[11] and Pheochromocytomas[13] have been identified.
Specific mutations in different splice sites in various genes causing breast cancer (e.g., BRCA1, PALB2), ovarian cancer (e.g., SLC9A3R1, COL7A1, HSD17B7), colon cancer (e.g., APC, MLH1, DPYD), colorectal cancer (e.g., COL3A1, APC, HLA-A), skin cancer (e.g., COL17A1, XPA, POLH), and Fanconi anemia (e.g., FANC, FANA) have been uncovered. The mutations in the donor and acceptor splice sites in different genes causing a variety of cancers that have been identified by S&S are shown in Table 1.
| Disease type | Gene symbol | Mutation location | Original sequence | Mutated sequence | Splicing aberration |
|---|---|---|---|---|---|
| Breast cancer | BRCA1 | Exon 11 | AAGGTGTGT | AAAGTGTGT | Skipping of exon 12[44] |
| PALB2 | Exon 12 | CAGGCAAGT | CAAGCAAGT | Potentially weakening the canonical donor splicing site[45] | |
| Ovarian cancer | SLC9A3R1 | Exon2 | GAGGTGATG | GAGGCGATG | Significant effect in ‘splicing’[12] |
| Colorectal Cancer | MLH1 | Exon 9 | TCGGTATGT | TCAGTATGT | Skipping of exon 8 and protein truncation[14] |
| MSH2 | Intron 8 | CAGGTATGC | CAGGCATGC | Intervening sequence, RNA processing,No amino acid change[14] | |
| MSH6 | Intron 9 | TTTTTAATTTTAAGG | TTTTTAATTTTGAGG | Intervening sequence, RNA processing,No amino acid change[14] | |
| Skin Cancer | TGFBR1 | Exon 5 | TTTTGATTCTTTAGG | TTTTGATTCTTTCGG | Exon 5 skipping[25] |
| ITGA6 | Intron 19 | TTATTTTCTAACAGG | TTATTTTCTAACACG | Skipping of the exon 20 and resulted in in-frame deletion[46] | |
| Birt–Hogg–Dubé (BHD) syndrome | FLCN | Exon 9 | GAAGTAAGC | GAAGGAAGC | Skipping of exon 9 and weak retention of 131 bp of intron 9[47] |
| Nevoid basal cell carcinoma | PTCH1 | Intron 4 | CAGGTATAT | CAGGTGTAT | Exon 4 Skipping [11] |
| Mesothelioma | BAP1 | Exon 16 | AAGGTGAGG | TAGGTGAGG | Creates a novel 5’ splice site that results in a 4 nucleotide deletion of the 3’ end of exon 16[48] |
Discovery of genes causing inherited disorders using S&S
Specific mutations in different splice sites in various genes that cause inherited disorders, including, for example, Type 1 diabetes (e.g., PTPN22, TCF1 (HCF-1A)), hypertension (e.g., LDL, LDLR, LPL), Marfan syndrome (e.g., FBN1, TGFBR2, FBN2), cardiac diseases (e.g., COL1A2, MYBPC3, ACTC1), eye disorders (e.g., EVC, VSX1) have been uncovered. A few example mutations in the donor and acceptor splice sites in different genes causing a variety of inherited disorders identified using S&S are shown in Table 2.
| Disease type | Gene symbol | Mutation location | Original sequence | Mutated sequence | Splicing aberration |
|---|---|---|---|---|---|
| Diabetes | PTPN22 | Exon 18 | AAGGTAAAG | AACGTAAAG | Skipping of exon 18[49] |
| TCF1 | Intron 4 | TTTGTGCCCCTCAGG | TTTGTGCCCCTCGGG | Skipping of exon 5[50] | |
| Hypertension | LDL | Intron 10 | TGGGTGCGT | TGGGTGCAT | Normolipidemic to classical heterozygous FH[51] |
| LDLR | Intron 2 | GCTGTGAGT | GCTGTGTGT | May cause splicing abnormalities through an in-silico analysis[52] | |
| LPL | Intron 2 | ACGGTAAGG | ACGATAAGG | Cryptic splice sites is activated in vivo at the sites[53] | |
| Marfan syndrome | FBN1 | Intron 46 | CAAGTAAGA | CAAGTAAAA | Exon skipping/cryptic splice site[54] |
| TGFBR2 | Intron 1 | ATCCTGTTTTACAGA | ATCCTGTTTTACGGA | Abnormal splicing[55] | |
| FBN2 | Intron45 | TGGGTAAGT | TGGGGAAGT | Splice site alterations leading to frameshift mutations,
causing a truncated protein[55] | |
| Cardiac disease | COL1A2 | Intron 46 | GCTGTAAGT | GCTGCAAGT | Permitted almost exclusive use of a cryptic donor
site 17 nt upstream in the exon[56] |
| MYBPC3 | Intron 5 | CTCCATGCACACAGG | CTCCATGCACACCGG | Abnormal mRNA transcript with a premature
stop codon will produce a truncated protein lacking the binding sites for myosin and titin[57] | |
| ACTC1 | Intron 1 | TTTTCTTCTCATAGG | TTTTCTTCTTATAGG | No effect [58] | |
| Eye disorder | ABCR | Intron 30 | CAGGTACCT | CAGTTACCT | Autosomal recessive RP and CRD[59] |
| VSX1 | Intron 5 | TTTTTTTTTACAAGG | TATTTTTTTACAAGG | Aberrant splicing[60] |
Genes causing immune system disorders
More than 100 immune system disorders affect humans, including inflammatory bowel diseases, multiple sclerosis, systemic lupus erythematosus, bloom syndrome, familial cold autoinflammatory syndrome, and dyskeratosis congenita. The Shapiro–Senapathy algorithm has been used to discover genes and mutations involved in many immune disorder diseases, including Ataxia telangiectasia, B-cell defects, epidermolysis bullosa, and X-linked agammaglobulinemia.
Xeroderma pigmentosum, an autosomal recessive disorder is caused by faulty proteins formed due to new preferred splice donor site identified using S&S algorithm and resulted in defective nucleotide excision repair.[31]
Type I Bartter syndrome (BS) is caused by mutations in the gene SLC12A1. S&S algorithm helped in disclosing the presence of two novel heterozygous mutations c.724 + 4A > G in intron 5 and c.2095delG in intron 16 leading to complete exon 5 skipping.[32]
Mutations in the MYH gene, which is responsible for removing the oxidatively damaged DNA lesion are cancer-susceptible in the individuals. The IVS1+5C plays a causative role in the activation of a cryptic splice donor site and the alternative splicing in intron 1, S&S algorithm shows, guanine (G) at the position of IVS+5 is well conserved (at the frequency of 84%) among primates. This also supported the fact that the G/C SNP in the conserved splice junction of the MYH gene causes the alternative splicing of intron 1 of the β type transcript.[33]
Splice site scores were calculated according to S&S to find EBV infection in X-linked lymphoproliferative disease.[61] Identification of Familial tumoral calcinosis (FTC) is an autosomal recessive disorder characterized by ectopic calcifications and elevated serum phosphate levels and it is because of aberrant splicing.[62]
Application of S&S in hospitals for clinical practice and research
Applying the S&S technology platform in modern clinical genomics research hasadvance diagnosis and treatment of human diseases.
In the modern era of Next Generation Sequencing (NGS) technology, S&S is applied in clinical practice extensively. Clinicians and molecular diagnostic laboratories apply S&S using various computational tools including HSF,[3] SSF,[4] and Alamut.[6] It is aiding in the discovery of genes and mutations in patients whose disease are stratified or when the disease in a patient is unknown based on clinical investigations.
In this context, S&S has been applied on cohorts of patients in different ethnic groups with various cancers and inherited disorders. A few examples are given below.
Cancers
| Cancer type | Publication title | Year | Ethnicity | Number of patients | |
|---|---|---|---|---|---|
| 1 | Breast cancer | The germline mutational landscape of BRCA1 and BRCA2 in Brazil[63] | 2018 | Brazil | 649 Patients |
| 2 | Hereditary non-polyposis colorectal cancer | Prevalence and characteristics of hereditary non-polyposis colorectal cancer (HNPCC) syndrome in immigrant Asian colorectal cancer patients[14] | 2017 | Asian Immigrant | 143 Patients |
| 3 | Nevoid basal cell carcinoma syndrome | Nevoid basal cell carcinoma syndrome caused by splicing mutations in the PTCH1 gene[11] | 2016 | Japanese | 10 Patients |
| 4 | Prostate cancer | Identification of Two Novel HOXB13 Germline Mutations in Portuguese Prostate Cancer Patients[64] | 2015 | Portuguese | 462 Patients, 132 Controls |
| 5 | Colorectal adenomatous polyposis | Identification of Novel Causative Genes for Colorectal Adenomatous Polyposis | 2015 | German | 181 Patients,531 Controls |
| 6 | Renal cell cancer | Genetic screening of the FLCN gene identify six novel variants and a Danish founder mutation[65] | 2016 | Danish | 143 individuals |
Inherited disorders
| Disease name | Publication title | Year | Ethnicity | Number of patients | |
|---|---|---|---|---|---|
| 1 | Bardet-Biedl Syndrome | The First Nationwide Survey and Genetic Analyses of Bardet-Biedl Syndrome in Japan[66] | 2015 | Japan | 38 Patients(Disease identified in 9 Patients) |
| 2 | Odontogenesis Diseases | Genetic Evidence Supporting the Role of the Calcium Channel, CACNA1S, in Tooth Cusp and Root Patterning[67] | 2018 | Thai families | 11 Patients,18 Controls |
| 3 | Beta-Ketothiolase Deficiency | Clinical and Mutational Characterizations of Ten Indian Patients with Beta-Ketothiolase Deficiency[68] | 2016 | Indian | 10 Patients |
| 4 | Unclear speech developmental delay | Progressive SCAR14 with unclear speech, developmental delay, tremor, and behavioral problems caused by a homozygous deletion of the SPTBN2 pleckstrin homology domain[69] | 2017 | Pakistani family | 9 Patients, 12 controls |
| 5 | Dent's disease | Dent's disease in children: diagnostic and therapeutic consideration[70] | 2015 | Poland | 10 Patients |
| 6 | Atypical Haemolytic Uraemic Syndrome | Genetics Atypical hemolytic-uremic syndrome[71] | 2015 | Newcastle cohort | 28 Families, 7 Sporadic patients |
| 7 | Age-related Macular Degeneration and Stargardt disease | Genetics of Age-related Macular Degeneration and Stargardt disease in South African populations[72] | 2015 | African Populations | 32 Patients |
S&S - the first algorithm for identifying splice sites, exons and split genes
Dr. Senapathy's original objective in developing a method for identifying splice sites was to find complete genes in raw uncharacterized genomic sequence that could be used in the human genome project.[73][2] In the landmark paper with this objective,[73] he described the basic method for identifying the splice sites within a given sequence based on the Position Weight Matrix (PWM)[1] of the splicing sequences in different eukaryotic organism groups for the first time. He also created the first exon detection method by defining the basic characteristics of an exon as the sequence bounded by an acceptor and a donor splice sites that had S&S scores above a threshold, and by an ORF that was mandatory for an exon. An algorithm for finding complete genes based on the identified exons was also described by Dr. Senapathy for the first time.[73][2]
Dr. Senapathy demonstrated that only deleterious mutations in the donor or acceptor splice sites that would drastically make the protein defective would reduce the splice site score (later known as the Shapiro–Senapathy score), and other non-deleterious variations would not reduce the score. The S&S method was adapted for researching the cryptic splice sites caused by mutations leading to diseases. This method for detecting deleterious splicing mutations in eukaryotic genes has been used extensively in disease research in the humans, animals and plants over the past three decades, as described above.
The basic method for splice site identification, and for defining exons and genes was subsequently used by researchers in finding splice sites, exons and eukaryotic genes in a variety of organisms. These methods also formed the basis of all subsequent tools development for discovering genes in uncharacterized genomic sequences. It also was used in a different computational approaches including machine learning and neural network, and in alternative splicing research.
Discovering the mechanisms of aberrant splicing in diseases
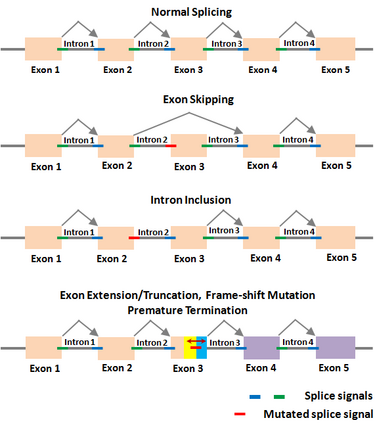
The Shapiro–Senapathy algorithm has been used to determine the various aberrant splicing mechanisms in genes due to deleterious mutations in the splice sites, which cause numerous diseases. Deleterious splice site mutations impair the normal splicing of the gene transcripts, and thereby make the encoded protein defective. A mutant splice site can become “weak” compared to the original site, due to which the mutated splice junction becomes unrecognizable by the spliceosomal machinery. This can lead to the skipping of the exon in the splicing reaction, resulting in the loss of that exon in the spliced mRNA (exon-skipping). On the other hand, a partial or complete intron could be included in the mRNA due to a splice site mutation that makes it unrecognizable (intron inclusion). A partial exon-skipping or intron inclusion can lead to premature termination of the protein from the mRNA, which will become defective leading to diseases. The S&S has thus paved the way to determine the mechanisms by which a deleterious mutation could lead to a defective protein, resulting in different diseases depending on which gene is affected.
Examples of splicing aberrations
| Disease type | Gene symbol | Mutation location | Original donor/acceptor | Mutated donor/acceptor | Aberration effect |
|---|---|---|---|---|---|
| Colon Cancer | APC | Intron 2 | AAGGTAGAT | AAGGAAGAT | Skipping of Exon 3[74] |
| Colorectal cancer | MSH2 | Exon 15 | GAGGTTTGT | GAGGTTTCT | Skipping of Exon 15[75] |
| Retinoblastoma | RB1 | Intron 23 | TCTTAACTTGACAGA | TCTTAACGTGACAGA | New splice acceptor, intron inclusion[23] |
| Trophic benign epidermolysis bullosa | COL17A1 | Intron 51 | AGCGTAAGT | AGCATAAGT | lead to exon skipping, intron inclusion, or the use of a cryptic splice site, resulting in either a truncated protein or a protein lacking a small region of the coding sequence[76] |
| Choroideremia | CHM | Intron 3 | CAGGTAAAG | CAGATAAAG | Premature termination codon[77] |
| Cowden syndrome | PTEN | Intron 4 | GAGGTAGGT | GAGATAGGT | Premature termination codon within exon 5[53] |
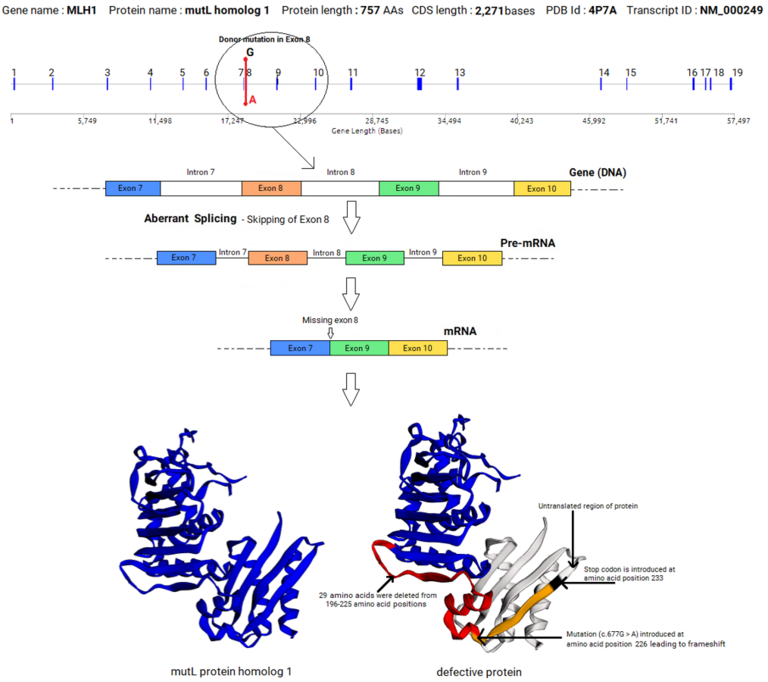
An example of splicing aberration (exon skipping) caused by a mutation in the donor splice site in the exon 8 of MLH1 gene that led to colorectal cancer is given below. This example shows that a mutation in a splice site within a gene can lead to a profound effect in the sequence and structure of the mRNA, and the sequence, structure and function of the encoded protein, leading to disease.
S&S in cryptic splice sites research and medical applications
The proper identification of splice sites has to be highly precise as the consensus splice sequences are very short and there are many other sequences similar to the authentic splice sites within gene sequences, which are known as cryptic, non-canonical, or pseudo splice sites. When an authentic or real splice site is mutated, any cryptic splice sites present close to the original real splice site could be erroneously used as authentic site, resulting in an aberrant mRNA. The erroneous mRNA may include a partial sequence from the neighboring intron or lose a partial exon, which may result in a premature stop codon. The result may be a truncated protein that would have lost its function completely.
Shapiro–Senapathy algorithm can identify the cryptic splice sites, in addition to the authentic splice sites. Cryptic sites can often be stronger than the authentic sites, with a higher S&S score. However, due to the lack of an accompanying complementary donor or acceptor site, this cryptic site will not be active or used in a splicing reaction. When a neighboring real site is mutated to become weaker than the cryptic site, then the cryptic site may be used instead of the real site, resulting in a cryptic exon and an aberrant transcript.
Numerous diseases have been caused by cryptic splice site mutations or usage of cryptic splice sites due to the mutations in authentic splice sites.[78][79][80][81][82]
S&S in animal and plant genomics research
S&S has also been used in RNA splicing research in many animals[83][84][85][86][87] and plants.[88][89][90][91][92]
The mRNA splicing plays a fundamental role in gene functional regulation. Very recently, it has been shown that A to G conversions at splice sites can lead to mRNA mis-splicing in Arabidopsis.[88] The splicing and exon–intron junction prediction coincided with the GT/AG rule (S&S) in the Molecular characterization and evolution of carnivorous sundew (Drosera rotundifolia L.) class V b-1,3-glucanase.[89] Unspliced (LSDH) and spliced (SSDH) transcripts of NAD+ dependent sorbitol dehydroge nase (NADSDH) of strawberry (Fragaria ananassa Duch., cv. Nyoho) were investigated for phytohormonal treatments.[90]
Ambra1 is a positive regulator of autophagy, a lysosome-mediated degradative process involved both in physiological and pathological conditions. Nowadays, this function of Ambra1 has been characterized only in mammals and zebrafish.[84] Diminution of rbm24a or rbm24b gene products by morpholino knockdown resulted in significant disruption of somite formation in mouse and zebrafish.[85] Dr.Senapathy algorithm used extensively to study intron-exon organization of fut8 genes. The intron-exon boundaries of Sf9 fut8 were in agreement with the consensus sequence for the splicing donor and acceptor sites concluded using S&S.[86]
The split-gene theory, introns and splice junctions
The motivation for Dr. Senapathy to develop a method for the detection of splice junctions came from his split-gene theory.[93] If primordial DNA sequences had a random nucleotide organization, the random distribution of stop codons would allow only very short Open Reading Frames (ORFs), as three stop codons out of 64 codons would result in an average ORF of ~60 bases. When Senapathy tested this in random DNA sequences, not only this was proven to be true, but the longest ORFs even in very long DNA sequences was found to be ~600 bases above which no ORFs existed. If so, a long coding sequence of even 1,200 bases (the average coding sequence length of genes from living organisms), and longer coding sequences of 6,000 bases (many of which occur in living organisms) will not occur in a primordial random sequence. Thus, genes had to occur in pieces in a split form, with short coding sequences (ORFs) that became exons, interrupted by very long random sequences that became introns. When the eukaryotic DNA was tested for ORF length distribution, it exactly matched that from random DNA, with very short ORFs that matched the lengths of exons, and very long introns as predicted, supporting the split gene theory.[93]
If this split gene theory was true, then the ends of these ORFs that had a stop codon by nature would have become the ends of exons that would occur within introns, and that would define the splice junctions. When this hypothesis was tested, the almost all splice junctions in eukaryotic genes were found to contain stop codons exactly at the ends of introns, bordering the exons.[94] In fact, these stop codons were found to form the “canonical” AG:GT splicing sequence, with the three stop codons occurring as part of the strong consensus signals. The Nobel Laureate Dr. Marshall Nirenberg, who deciphered the codons, stated that these findings strongly showed that the split gene theory for the origin of introns and the split structure of genes must be valid, and communicated the paper to the PNAS.[93] New Scientist covered this publication in “A long explanation for introns”.[95]
This basic split gene theory led to the hypothesis that the splice junctions originated from the stop codons.[94] Besides the codon CAG, only TAG, which is a stop codon, was found at the ends of introns. Surprisingly, all three stop codons (TGA, TAA and TAG) were found after one base (G) at the start of introns. These stop codons are shown in the consensus canonical donor splice junction as AG:GT(A/G)GGT, wherein the TAA and TGA are the stop codons, and the additional TAG is also present at this position. The canonical acceptor splice junction is shown as (C/T)AG:GT, in which TAG is the stop codon. These consensus sequence clearly show the presence of the stop codons at the ends of introns bordering the exons in all eukaryotic genes. Dr. Marshall Nirenberg again stated that these observations fully supported the split gene theory for the origin of splice junction sequences from stop codons, who was the referee for this paper.[94] New Scientist covered this publication in “Exons, Introns and Evolution”.[96]
Dr. Senapathy wanted to detect the splice junctions in random DNA based on the consensus splice signal sequences, as he found that there were many sequences resembling splice sites that were not the real splice sites within genes.[94][73][2] This Position Weight Matrix method turned out to be a highly accurate algorithm to detect the real splice sites and the cryptic sites in genes. He also formulated the first exon detection method, based on the requirement for splice junctions at the ends of exons, and the requirement for an Open Reading Frame that would contain the exon.[73][2] This exon detection method also turned to be highly accurate, detecting most of the exons with few false positives and false negatives. He extended this approach to define a complete split gene in a eukaryotic genomic sequence.[73][2] Thus, the PWM based algorithm turned out to be very sensitive to not only detect the real splice sites and cryptic sites, but also to detect mutated splice sites that are deleterious as opposed to non-deleterious splicing mutations.
The stop codons within splice junctions turned out to be the strongest bases in splice junctions of eukaryotic genes, when tested using the PWMs of the consensus sequences.[73][2] In fact, it was shown that mutations in these bases were the cause of diseases compared to other bases, as these three of the four bases (base 1, 3 and 4) of the canonical AG:GT were part of the stop codons. Senapathy showed that, when these canonical bases were mutated, the splice site score became weak, causing splicing aberrations in the splicing process and translation of the mRNA (as described under the diseases section above). Although the value of the splice site detection method in discovering genes with splicing mutations that caused disease has been realized over the years, its importance in clinical medicine is increasingly realized in the Next Generation Sequencing era over the past five years, with its incorporation in several tools based on the S&S algorithm.[97]
Dr. Senapathy is currently the President and CSO of Genome International Corporation (GIC), a genomics R&D company based in Madison, WI. His team has developed several databases and tools for the analysis of splice junctions, including EuSplice,[98] AspAlt,[99] ExDom[100] and RoBust.[101] AspAlt was commended by Biotechniques, which stated that it solved a difficult problem for scientists in the comparative analysis and visualization of alternative splicing across different genomes.[102] GIC has most recently developed the clinical genomics analysis platform Genome Explorer®.
Selected publications
- Shapiro, Marvin B.; Senapathy, Periannan (1987). "RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression". Nucleic Acids Research 15 (17): 7155–7174. doi:10.1093/nar/15.17.7155. PMID 3658675.
- Senapathy, P. (1988). "Possible evolution of splice-junction signals in eukaryotic genes from stop codons". Proc Natl Acad Sci U S A 85 (4): 1129–33. doi:10.1073/pnas.85.4.1129. PMID 3422483. Bibcode: 1988PNAS...85.1129S.
- Senapathy, P; Shapiro, MB; Harris, NL (1990). "Splice junctions, branch point sites, and exons: sequence statistics, identification, and applications to genome project.". Methods in Enzymology 183: 252–78. doi:10.1016/0076-6879(90)83018-5. PMID 2314278.
- Harris, N.L.; Senapathy, P. (1990). "Distribution and consensus of branch point signals in eukaryotic genes: a computerized statistical analysis". Nucleic Acids Res. 18 (10): 3015–9. doi:10.1093/nar/18.10.3015. PMID 2349097.
- Senapathy, P. (1986). "Origin of eukaryotic introns: a hypothesis, based on codon distribution statistics in genes, and its implications". Proc Natl Acad Sci U S A 83 (7): 2133–7. doi:10.1073/pnas.83.7.2133. PMID 3457379. Bibcode: 1986PNAS...83.2133S.
- Regulapati, R.; Bhasi, A.; Singh, C.K.; Senapathy, P. (2008). "Origination of the Split Structure of Spliceosomal Genes from Random Genetic Sequences". PLOS ONE 3 (10): 10. doi:10.1371/journal.pone.0003456. PMID 18941625. Bibcode: 2008PLoSO...3.3456R.
- Senapathy, P. (1995). "Introns and the origin of protein-coding genes". Science 268 (5215): 1366–7. doi:10.1126/science.7761858. PMID 7761858. Bibcode: 1995Sci...268.1366S.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Shapiro, Marvin B.; Senapathy, Periannan (1987). "RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression". Nucleic Acids Research 15 (17): 7155–7174. doi:10.1093/nar/15.17.7155. ISSN 0305-1048. PMID 3658675.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Senapathy, Periannan; Shapiro, Marvin B.; Harris, Nomi L. (1990), "[16 Splice junctions, branch point sites, and exons: Sequence statistics, identification, and applications to genome project"], Methods in Enzymology (Elsevier) 183: 252–278, doi:10.1016/0076-6879(90)83018-5, ISBN 9780121820848, PMID 2314278, https://archive.org/details/molecularevoluti0000unse_d5p9/page/252
- ↑ 3.0 3.1 Desmet, François-Olivier; Hamroun, Dalil; Lalande, Marine; Collod-Béroud, Gwenaëlle; Claustres, Mireille; Béroud, Christophe (2009-04-01). "Human Splicing Finder: an online bioinformatics tool to predict splicing signals". Nucleic Acids Research 37 (9): e67. doi:10.1093/nar/gkp215. ISSN 1362-4962. PMID 19339519.
- ↑ 4.0 4.1 "Splice-Site Analyzer Tool". http://ibis.tau.ac.il/ssat/SpliceSiteFrame.htm.
- ↑ Buratti, E.; Chivers, M.; Hwang, G.; Vorechovsky, I. (2010-10-06). "DBASS3 and DBASS5: databases of aberrant 3'- and 5'-splice sites". Nucleic Acids Research 39 (Database): D86–D91. doi:10.1093/nar/gkq887. ISSN 0305-1048. PMID 20929868.
- ↑ 6.0 6.1 Houdayer, Claude (2011), "In Silico Prediction of Splice-Affecting Nucleotide Variants", In Silico Tools for Gene Discovery, Methods in Molecular Biology, 760, Humana Press, pp. 269–281, doi:10.1007/978-1-61779-176-5_17, ISBN 9781617791758, PMID 21780003
- ↑ Schwartz, S.; Hall, E.; Ast, G. (2009-05-08). "SROOGLE: webserver for integrative, user-friendly visualization of splicing signals". Nucleic Acids Research 37 (Web Server): W189–W192. doi:10.1093/nar/gkp320. ISSN 0305-1048. PMID 19429896.
- ↑ Damiola, Francesca; Schultz, Inès; Barjhoux, Laure; Sornin, Valérie; Dondon, Marie-Gabrielle; Eon-Marchais, Séverine; Marcou, Morgane; Caron, Olivier et al. (2015-11-12). "Mutation analysis of PALB2 gene in French breast cancer families". Breast Cancer Research and Treatment 154 (3): 463–471. doi:10.1007/s10549-015-3625-7. ISSN 0167-6806. PMID 26564480.
- ↑ Lara, Karlena; Consigliere, Nigmet; Pérez, Jorge; Porco, Antonietta (January 2012). "BRCA1 and BRCA2mutations in breast cancer patients from Venezuela". Biological Research 45 (2): 117–130. doi:10.4067/S0716-97602012000200003. ISSN 0716-9760. PMID 23096355.
- ↑ Mucaki, Eliseos J.; Caminsky, Natasha G.; Perri, Ami M.; Lu, Ruipeng; Laederach, Alain; Halvorsen, Matthew; Knoll, Joan H. M.; Rogan, Peter K. (2016-04-11). "A unified analytic framework for prioritization of non-coding variants of uncertain significance in heritable breast and ovarian cancer". BMC Medical Genomics 9 (1): 19. doi:10.1186/s12920-016-0178-5. ISSN 1755-8794. PMID 27067391.
- ↑ 11.0 11.1 11.2 11.3 Kato, Chise; Fujii, Kentaro; Arai, Yuto; Hatsuse, Hiromi; Nagao, Kazuaki; Takayama, Yoshinaga; Kameyama, Kouzou; Fujii, Katsunori et al. (2016-08-25). "Nevoid basal cell carcinoma syndrome caused by splicing mutations in the PTCH1 gene". Familial Cancer 16 (1): 131–138. doi:10.1007/s10689-016-9924-2. ISSN 1389-9600. PMID 27561271.
- ↑ 12.0 12.1 KREIMANN, ERICA LORENA; RATAJSKA, MAGDALENA; KUZNIACKA, ALINA; DEMACOPULO, BRENDA; STUKAN, MACIEJ; LIMON, JANUSZ (2015-10-12). "A novel splicing mutation in the SLC9A3R1 gene in tumors from ovarian cancer patients". Oncology Letters 10 (6): 3722–3726. doi:10.3892/ol.2015.3796. ISSN 1792-1074. PMID 26788197.
- ↑ 13.0 13.1 Welander, Jenny; Larsson, Catharina; Bäckdahl, Martin; Hareni, Niyaz; Sivlér, Tobias; Brauckhoff, Michael; Söderkvist, Peter; Gimm, Oliver (2012-09-24). "Integrative genomics reveals frequent somatic NF1 mutations in sporadic pheochromocytomas". Human Molecular Genetics 21 (26): 5406–5416. doi:10.1093/hmg/dds402. ISSN 1460-2083. PMID 23010473.
- ↑ 14.0 14.1 14.2 14.3 14.4 Lee, Jasmine; Xiao, Yin-Yi; Sun, Yan Yu; Balderacchi, Jasminka; Clark, Bradley; Desani, Jatin; Kumar, Vivek; Saverimuthu, Angela et al. (December 2017). "Prevalence and characteristics of hereditary non-polyposis colorectal cancer (HNPCC) syndrome in immigrant Asian colorectal cancer patients". BMC Cancer 17 (1): 843. doi:10.1186/s12885-017-3799-y. ISSN 1471-2407. PMID 29237405.
- ↑ 15.0 15.1 Dudley, Beth; Brand, Randall E.; Thull, Darcy; Bahary, Nathan; Nikiforova, Marina N.; Pai, Reetesh K. (August 2015). "Germline MLH1 Mutations Are Frequently Identified in Lynch Syndrome Patients With Colorectal and Endometrial Carcinoma Demonstrating Isolated Loss of PMS2 Immunohistochemical Expression". The American Journal of Surgical Pathology 39 (8): 1114–1120. doi:10.1097/pas.0000000000000425. ISSN 0147-5185. PMID 25871621.
- ↑ Mensenkamp, Arjen R.; Vogelaar, Ingrid P.; van Zelst–Stams, Wendy A.G.; Goossens, Monique; Ouchene, Hicham; Hendriks–Cornelissen, Sandra J.B.; Kwint, Michael P.; Hoogerbrugge, Nicoline et al. (March 2014). "Somatic Mutations in MLH1 and MSH2 Are a Frequent Cause of Mismatch-Repair Deficiency in Lynch Syndrome-Like Tumors". Gastroenterology 146 (3): 643–646.e8. doi:10.1053/j.gastro.2013.12.002. ISSN 0016-5085. PMID 24333619.
- ↑ Eggington, J.M.; Bowles, K.R.; Moyes, K.; Manley, S.; Esterling, L.; Sizemore, S.; Rosenthal, E.; Theisen, A. et al. (2013-12-20). "A comprehensive laboratory-based program for classification of variants of uncertain significance in hereditary cancer genes". Clinical Genetics 86 (3): 229–237. doi:10.1111/cge.12315. ISSN 0009-9163. PMID 24304220.
- ↑ Toki, Tsutomu; Kanezaki, Rika; Kobayashi, Eri; Kaneko, Hiroshi; Suzuki, Mikiko; Wang, RuNan; Terui, Kiminori; Kanegane, Hirokazu et al. (2013-04-18). "Naturally occurring oncogenic GATA1 mutants with internal deletions in transient abnormal myelopoiesis in Down syndrome". Blood 121 (16): 3181–3184. doi:10.1182/blood-2012-01-405746. ISSN 0006-4971. PMID 23440243.
- ↑ Hildebrand, Michael S.; Tankard, Rick; Gazina, Elena V.; Damiano, John A.; Lawrence, Kate M.; Dahl, Hans-Henrik M.; Regan, Brigid M.; Shearer, Aiden Eliot et al. (2015-07-03). "PRIMA1mutation: a new cause of nocturnal frontal lobe epilepsy". Annals of Clinical and Translational Neurology 2 (8): 821–830. doi:10.1002/acn3.224. ISSN 2328-9503. PMID 26339676.
- ↑ van Kuilenburg, André B. P.; Meijer, Judith; Mul, Adri N. P. M.; Meinsma, Rutger; Schmid, Veronika; Dobritzsch, Doreen; Hennekam, Raoul C. M.; Mannens, Marcel M. A. M. et al. (2010-08-29). "Intragenic deletions and a deep intronic mutation affecting pre-mRNA splicing in the dihydropyrimidine dehydrogenase gene as novel mechanisms causing 5-fluorouracil toxicity". Human Genetics 128 (5): 529–538. doi:10.1007/s00439-010-0879-3. ISSN 0340-6717. PMID 20803296.
- ↑ Wittler, Lars; Hilger, Alina; Proske, Judith; Pennimpede, Tracie; Draaken, Markus; Ebert, Anne-Karoline; Rösch, Wolfgang; Stein, Raimund et al. (September 2012). "Murine expression and mutation analyses of the prostate androgen-regulated mucin-like protein 1 (Parm1) gene, a candidate for human epispadias". Gene 506 (2): 392–395. doi:10.1016/j.gene.2012.06.082. ISSN 0378-1119. PMID 22766399.
- ↑ Nishida, Atsushi; Minegishi, Maki; Takeuchi, Atsuko; Niba, Emma Tabe Eko; Awano, Hiroyuki; Lee, Tomoko; Iijima, Kazumoto; Takeshima, Yasuhiro et al. (2015-04-02). "Tissue- and case-specific retention of intron 40 in mature dystrophin mRNA". Journal of Human Genetics 60 (6): 327–333. doi:10.1038/jhg.2015.24. ISSN 1434-5161. PMID 25833469.
- ↑ 23.0 23.1 Zhang, Katherine; Nowak, Inga; Rushlow, Diane; Gallie, Brenda L.; Lohmann, Dietmar R. (2008-01-07). "Patterns of missplicing caused byRB1gene mutations in patients with retinoblastoma and association with phenotypic expression". Human Mutation 29 (4): 475–484. doi:10.1002/humu.20664. ISSN 1059-7794. PMID 18181215.
- ↑ Hung, Chia-Cheng; Lin, Shin-Yu; Lee, Chien-Nan; Chen, Chih-Ping; Lin, Shuan-Pei; Chao, Mei-Chyn; Chiou, Shyh-Shin; Su, Yi-Ning (2011-05-26). "Low penetrance of retinoblastoma for p.V654L mutation of the RB1 gene". BMC Medical Genetics 12 (1): 76. doi:10.1186/1471-2350-12-76. ISSN 1471-2350. PMID 21615945.
- ↑ 25.0 25.1 25.2 Fujiwara, Takayuki; Takeda, Norifumi; Hara, Hironori; Morita, Hiroyuki; Kishihara, Jun; Inuzuka, Ryo; Yagi, Hiroki; Maemura, Sonoko et al. (2018-04-30). "Distinct variants affecting differential splicing of TGFBR1 exon 5 cause either Loeys–Dietz syndrome or multiple self-healing squamous epithelioma". European Journal of Human Genetics 26 (8): 1151–1158. doi:10.1038/s41431-018-0127-1. ISSN 1018-4813. PMID 29706644.
- ↑ Morrison, Arianne; Chekaluk, Yvonne; Bacares, Ruben; Ladanyi, Marc; Zhang, Liying (2015-04-01). "BAP1 Missense Mutation c.2054 A>T (p.E685V) Completely Disrupts Normal Splicing through Creation of a Novel 5' Splice Site in a Human Mesothelioma Cell Line". PLOS ONE 10 (4): e0119224. doi:10.1371/journal.pone.0119224. ISSN 1932-6203. PMID 25830670. Bibcode: 2015PLoSO..1019224M.
- ↑ Richter, Toni M; Tong, Benton D; Scholnick, Steven B (2005). "Epigenetic inactivation and aberrant transcription of CSMD1 in squamous cell carcinoma cell lines". Cancer Cell International 5 (1): 29. doi:10.1186/1475-2867-5-29. ISSN 1475-2867. PMID 16153303.
- ↑ 28.0 28.1 van der Post, Rachel S.; Vogelaar, Ingrid P.; Manders, Peggy; van der Kolk, Lizet E.; Cats, Annemieke; van Hest, Liselotte P.; Sijmons, Rolf; Aalfs, Cora M. et al. (October 2015). "Accuracy of Hereditary Diffuse Gastric Cancer Testing Criteria and Outcomes in Patients With a Germline Mutation in CDH1". Gastroenterology 149 (4): 897–906.e19. doi:10.1053/j.gastro.2015.06.003. ISSN 0016-5085. PMID 26072394.
- ↑ ZHU, MING; CHEN, HUI-MEI; WANG, YA-PING (2013-03-11). "Missense mutations of MLH1 and MSH2 genes detected in patients with gastrointestinal cancer are associated with exonic splicing enhancers and silencers". Oncology Letters 5 (5): 1710–1718. doi:10.3892/ol.2013.1243. ISSN 1792-1074. PMID 23760103.
- ↑ Castiglia, Daniele; Pagani, Elena; Alvino, Ester; Vernole, Patrizia; Marra, Giancarlo; Cannavò, Elda; Jiricny, Josef; Zambruno, Giovanna et al. (June 2003). "Biallelic somatic inactivation of the mismatch repair gene MLH1 in a primary skin melanoma". Genes, Chromosomes and Cancer 37 (2): 165–175. doi:10.1002/gcc.10193. ISSN 1045-2257. PMID 12696065.
- ↑ 31.0 31.1 Sidwell, R.U.; Sandison, A.; Wing, J.; Fawcett, H.D.; Seet, J-E.; Fisher, C.; Nardo, T.; Stefanini, M. et al. (July 2006). "A novel mutation in the XPA gene associated with unusually mild clinical features in a patient who developed a spindle cell melanoma". British Journal of Dermatology 155 (1): 81–88. doi:10.1111/j.1365-2133.2006.07272.x. ISSN 0007-0963. PMID 16792756.
- ↑ 32.0 32.1 Nozu, Kandai; Iijima, Kazumoto; Kawai, Kazuo; Nozu, Yoshimi; Nishida, Atsushi; Takeshima, Yasuhiro; Fu, Xue Jun; Hashimura, Yuya et al. (10 July 2009). "In vivo and in vitro splicing assay of SLC12A1 in an antenatal salt-losing tubulopathy patient with an intronic mutation". Human Genetics 126 (4): 533–538. doi:10.1007/s00439-009-0697-7. ISSN 0340-6717. PMID 19513753.
- ↑ 33.0 33.1 Yamaguchi, Satoru; Shinmura, Kazuya; Saitoh, Takayuki; Takenoshita, Seiichi; Kuwano, Hiroyuki; Yokota, Jun (May 2002). "A single nucleotide polymorphism at the splice donor site of the human MYH base excision repair genes results in reduced translation efficiency of its transcripts". Genes to Cells: Devoted to Molecular & Cellular Mechanisms 7 (5): 461–474. doi:10.1046/j.1365-2443.2002.00532.x. ISSN 1356-9597. PMID 12056405.
- ↑ Lee, Jasmine; Xiao, Yin-Yi; Sun, Yan Yu; Balderacchi, Jasminka; Clark, Bradley; Desani, Jatin; Kumar, Vivek; Saverimuthu, Angela et al. (December 2017). "Prevalence and characteristics of hereditary non-polyposis colorectal cancer (HNPCC) syndrome in immigrant Asian colorectal cancer patients". BMC Cancer 17 (1): 843. doi:10.1186/s12885-017-3799-y. ISSN 1471-2407. PMID 29237405.
- ↑ Moles-Fernández, Alejandro; Duran-Lozano, Laura; Montalban, Gemma; Bonache, Sandra; López-Perolio, Irene; Menéndez, Mireia; Santamariña, Marta; Behar, Raquel et al. (2018). "Computational Tools for Splicing Defect Prediction in Breast/Ovarian Cancer Genes: How Efficient Are They at Predicting RNA Alterations?" (in en). Frontiers in Genetics 9: 366. doi:10.3389/fgene.2018.00366. ISSN 1664-8021. PMID 30233647.
- ↑ Zhang, Sidi; Samocha, Kaitlin E.; Rivas, Manuel A.; Karczewski, Konrad J.; Daly, Emma; Schmandt, Ben; Neale, Benjamin M.; MacArthur, Daniel G. et al. (2018-07-01). "Base-specific mutational intolerance near splice sites clarifies the role of nonessential splice nucleotides". Genome Research 28 (7): 968–974. doi:10.1101/gr.231902.117. ISSN 1088-9051. PMID 29858273.
- ↑ Bayés, M.; Hartung, A. J.; Ezer, S.; Pispa, J.; Thesleff, I.; Srivastava, A. K.; Kere, J. (October 1998). "The anhidrotic ectodermal dysplasia gene (EDA) undergoes alternative splicing and encodes ectodysplasin-A with deletion mutations in collagenous repeats". Human Molecular Genetics 7 (11): 1661–1669. doi:10.1093/hmg/7.11.1661. ISSN 0964-6906. PMID 9736768.
- ↑ Ars, E. (2000-01-22). "Mutations affecting mRNA splicing are the most common molecular defects in patients with neurofibromatosis type 1". Human Molecular Genetics 9 (2): 237–247. doi:10.1093/hmg/9.2.237. ISSN 1460-2083. PMID 10607834.
- ↑ Ars, E.; Kruyer, H.; Morell, M.; Pros, E.; Serra, E.; Ravella, A.; Estivill, X.; Lázaro, C. (2003-06-01). "Recurrent mutations in the NF1 gene are common among neurofibromatosis type 1 patients". Journal of Medical Genetics 40 (6): e82. doi:10.1136/jmg.40.6.e82. ISSN 0022-2593. PMID 12807981.
- ↑ Kiyozumi, Yoshimi; Matsubayashi, Hiroyuki; Horiuchi, Yasue; Oishi, Takuma; Abe, Masato; Ohnami, Sumiko; Naruoka, Akane; Kusuhara, Masatoshi et al. (2018-04-23). "A novel MLH1 intronic variant in a young Japanese patient with Lynch syndrome". Human Genome Variation 5 (1): 3. doi:10.1038/s41439-018-0002-1. ISSN 2054-345X. PMID 29760937.
- ↑ Humar, Bostjan; Toro, Tumi; Graziano, Francesco; Müller, Hansjakob; Dobbie, Zuzana; Kwang-Yang, Han; Eng, Charis; Hampel, Heather et al. (May 2002). "Novel germline CDH1 mutations in hereditary diffuse gastric cancer families". Human Mutation 19 (5): 518–525. doi:10.1002/humu.10067. ISSN 1098-1004. PMID 11968084.
- ↑ Becker, A. J.; Löbach, M.; Klein, H.; Normann, S.; Nöthen, M. M.; von Deimling, A.; Mizuguchi, M.; Elger, C. E. et al. (March 2001). "Mutational analysis of TSC1 and TSC2 genes in gangliogliomas". Neuropathology and Applied Neurobiology 27 (2): 105–114. doi:10.1046/j.0305-1846.2001.00302.x. ISSN 0305-1846. PMID 11437991.
- ↑ Schick, Volker; Majores, Michael; Engels, Gudrun; Spitoni, Sylvia; Koch, Arend; Elger, Christian E.; Simon, Matthias; Knobbe, Christiane et al. (2006-09-30). "Activation of Akt independent of PTEN and CTMP tumor-suppressor gene mutations in epilepsy-associated Taylor-type focal cortical dysplasias". Acta Neuropathologica 112 (6): 715–725. doi:10.1007/s00401-006-0128-y. ISSN 0001-6322. PMID 17013611.
- ↑ Ashton-Prolla, Patricia; Weitzel, Jeffrey N.; Herzog, Josef; Nogueira, Sonia Tereza dos Santos; Miguel, Diego; Bernardi, Pricila; Schwartz, Ida V. D.; Cintra, Terezinha Sarquis et al. (2018-06-15). "The germline mutational landscape of BRCA1 and BRCA 2 in Brazil". Scientific Reports 8 (1): 9188. doi:10.1038/s41598-018-27315-2. ISSN 2045-2322. PMID 29907814. Bibcode: 2018NatSR...8.9188P.
- ↑ Muller, Danièle; Mazoyer, Sylvie; Stoppa-Lyonnet, Dominique; Sinilnikova, Olga M.; Andrieu, Nadine; Fricker, Jean-Pierre; Bignon, Yves-Jean; Longy, Michel et al. (2015-12-01). "Mutation analysis of PALB2 gene in French breast cancer families". Breast Cancer Research and Treatment 154 (3): 463–471. doi:10.1007/s10549-015-3625-7. ISSN 1573-7217. PMID 26564480.
- ↑ Masunaga, Takuji; Ogawa, Junki; Akiyama, Masashi; Nishikawa, Takeji; Shimizu, Hiroshi; Ishiko, Akira (2017). "Compound heterozygosity for novel splice site mutations of ITGA6 in lethal junctional epidermolysis bullosa with pyloric atresia". The Journal of Dermatology 44 (2): 160–166. doi:10.1111/1346-8138.13575. ISSN 1346-8138. PMID 27607025.
- ↑ Hansen, Thomas vO; Nielsen, Finn C.; Gerdes, Anne-Marie; Ousager, Lilian B.; Jensen, Uffe B.; Skytte, Anne-Bine; Albrechtsen, Anders; Rossing, Maria (February 2017). "Genetic screening of the FLCN gene identify six novel variants and a Danish founder mutation". Journal of Human Genetics 62 (2): 151–157. doi:10.1038/jhg.2016.118. ISSN 1435-232X. PMID 27734835.
- ↑ Zhang, Liying; Ladanyi, Marc; Bacares, Ruben; Chekaluk, Yvonne; Morrison, Arianne (2015-04-01). "BAP1 Missense Mutation c.2054 A>T (p.E685V) Completely Disrupts Normal Splicing through Creation of a Novel 5' Splice Site in a Human Mesothelioma Cell Line". PLOS ONE 10 (4): e0119224. doi:10.1371/journal.pone.0119224. ISSN 1932-6203. PMID 25830670. Bibcode: 2015PLoSO..1019224M.
- ↑ Onengut-Gumuscu, Suna; Buckner, Jane H.; Concannon, Patrick (2006-10-01). "A Haplotype-Based Analysis of the PTPN22 Locus in Type 1 Diabetes". Diabetes 55 (10): 2883–2889. doi:10.2337/db06-0225. ISSN 0012-1797. PMID 17003357. http://diabetes.diabetesjournals.org/content/55/10/2883.
- ↑ Kralovicova, J.; Christensen, M. B.; Vorechovsky, I. (2005-09-01). "Biased exon/intron distribution of cryptic and de novo 3' splice sites". Nucleic Acids Research 33 (15): 4882–4898. doi:10.1093/nar/gki811. ISSN 0305-1048. PMID 16141195.
- ↑ Jensen, Hk; Jensen, Lg; Holst, Hu; Andreasen, Ph; Hansen, Ps; Larsen, Ml; Kolvraa, S; Bolund, L et al. (November 1999). "Normolipidemia and hypercholesterolemia in persons heterozygous for the same 1592+5GA splice site mutation in the low-density lipoprotein receptor gene". Clinical Genetics 56 (5): 379–389. doi:10.1034/j.1399-0004.1999.560506.x. ISSN 0009-9163. PMID 10668928.
- ↑ Al-Khateeb, Alyaa; Zahri, Mohd K; Mohamed, Mohd S; Sasongko, Teguh H; Ibrahim, Suhairi; Yusof, Zurkurnai; Zilfalil, Bin A (2011-03-19). "Analysis of sequence variations in low-density lipoprotein receptor gene among Malaysian patients with familial hypercholesterolemia". BMC Medical Genetics 12 (1): 40. doi:10.1186/1471-2350-12-40. ISSN 1471-2350. PMID 21418584.
- ↑ 53.0 53.1 Roca, X. (2003-11-01). "Intrinsic differences between authentic and cryptic 5' splice sites". Nucleic Acids Research 31 (21): 6321–6333. doi:10.1093/nar/gkg830. ISSN 1362-4962. PMID 14576320.
- ↑ Nijbroek, G.; Sood, S.; McIntosh, I.; Francomano, C. A.; Bull, E.; Pereira, L.; Ramirez, F.; Pyeritz, R. E. et al. (July 1995). "Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons". American Journal of Human Genetics 57 (1): 8–21. ISSN 0002-9297. PMID 7611299.
- ↑ 55.0 55.1 Frederic, Melissa Yana; Hamroun, Dalil; Faivre, Laurence; Boileau, Catherine; Jondeau, Guillaume; Claustres, Mireille; Béroud, Christophe; Collod-Béroud, Gwenaëlle (January 2008). "A new locus-specific database (LSDB) for mutations in theTGFBR2gene: UMD-TGFBR2". Human Mutation 29 (1): 33–38. doi:10.1002/humu.20602. ISSN 1059-7794. PMID 17935258. https://www.hal.inserm.fr/inserm-00343980/file/2008-HumMut%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20%20HAL%20%20%20%20%20%20%20%20%20%20%20%20%20UMD-TGFBR2.pdf.
- ↑ Schwarze, Ulrike; Hata, Ryu-Ichiro; McKusick, Victor A.; Shinkai, Hiroshi; Hoyme, H. Eugene; Pyeritz, Reed E.; Byers, Peter H. (May 2004). "Rare Autosomal Recessive Cardiac Valvular Form of Ehlers-Danlos Syndrome Results from Mutations in the COL1A2 Gene That Activate the Nonsense-Mediated RNA Decay Pathway". The American Journal of Human Genetics 74 (5): 917–930. doi:10.1086/420794. ISSN 0002-9297. PMID 15077201.
- ↑ Jääskeläinen, Pertti; Kuusisto, Johanna; Miettinen, Raija; Kärkkäinen, Päivi; Kärkkäinen, Satu; Heikkinen, Sami; Peltola, Paula; Pihlajamäki, Jussi et al. (4 November 2002). "Mutations in the cardiac myosin-binding protein C gene are the predominant cause of familial hypertrophic cardiomyopathy in eastern Finland". Journal of Molecular Medicine 80 (7): 412–422. doi:10.1007/s00109-002-0323-9. ISSN 0946-2716. PMID 12110947.
- ↑ Attanasio, M; Lapini, I; Evangelisti, L; Lucarini, L; Giusti, B; Porciani, MC; Fattori, R; Anichini, C et al. (2008-04-23). "FBN1 mutation screening of patients with Marfan syndrome and related disorders: detection of 46 novel FBN1 mutations". Clinical Genetics 74 (1): 39–46. doi:10.1111/j.1399-0004.2008.01007.x. ISSN 0009-9163. PMID 18435798.
- ↑ Cremers, F. (1998-03-01). "Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR". Human Molecular Genetics 7 (3): 355–362. doi:10.1093/hmg/7.3.355. ISSN 1460-2083. PMID 9466990.
- ↑ Dash, D P; George, S; O'Prey, D; Burns, D; Nabili, S; Donnelly, U; Hughes, A E; Silvestri, G et al. (2009-09-18). "Mutational screening of VSX1 in keratoconus patients from the European population". Eye 24 (6): 1085–1092. doi:10.1038/eye.2009.217. ISSN 0950-222X. PMID 19763142.
- ↑ Coffey, Alison J.; Brooksbank, Robert A.; Brandau, Oliver; Oohashi, Toshitaka; Howell, Gareth R.; Bye, Jacqueline M.; Cahn, Anthony P.; Durham, Jillian et al. (October 1998). "Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene". Nature Genetics 20 (2): 129–135. doi:10.1038/2424. ISSN 1061-4036. PMID 9771704.
- ↑ Benet-Pagès, Anna; Orlik, Peter; Strom, Tim M.; Lorenz-Depiereux, Bettina (2004-12-08). "An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia". Human Molecular Genetics 14 (3): 385–390. doi:10.1093/hmg/ddi034. ISSN 1460-2083. PMID 15590700.
- ↑ Palmero, Edenir Inêz; Carraro, Dirce Maria; Alemar, Barbara; Moreira, Miguel Angelo Martins; Ribeiro-dos-Santos, Ândrea; Abe-Sandes, Kiyoko; Galvão, Henrique Campos Reis; Reis, Rui Manuel et al. (2018-06-15). "The germline mutational landscape of BRCA1 and BRCA2 in Brazil". Scientific Reports 8 (1): 9188. doi:10.1038/s41598-018-27315-2. ISSN 2045-2322. PMID 29907814. Bibcode: 2018NatSR...8.9188P.
- ↑ Maia, Sofia; Cardoso, Marta; Pinto, Pedro; Pinheiro, Manuela; Santos, Catarina; Peixoto, Ana; Bento, Maria José; Oliveira, Jorge et al. (2015-07-15). "Identification of Two Novel HOXB13 Germline Mutations in Portuguese Prostate Cancer Patients". PLOS ONE 10 (7): e0132728. doi:10.1371/journal.pone.0132728. ISSN 1932-6203. PMID 26176944. Bibcode: 2015PLoSO..1032728M.
- ↑ Rossing, Maria; Albrechtsen, Anders; Skytte, Anne-Bine; Jensen, Uffe B; Ousager, Lilian B; Gerdes, Anne-Marie; Nielsen, Finn C; Hansen, Thomas vO (2016-10-13). "Genetic screening of the FLCN gene identify six novel variants and a Danish founder mutation". Journal of Human Genetics 62 (2): 151–157. doi:10.1038/jhg.2016.118. ISSN 1434-5161. PMID 27734835.
- ↑ Hirano, Makito; Satake, Wataru; Ihara, Kenji; Tsuge, Ikuya; Kondo, Shuji; Saida, Ken; Betsui, Hiroyuki; Okubo, Kazuhiro et al. (2015-09-01). "The First Nationwide Survey and Genetic Analyses of Bardet-Biedl Syndrome in Japan". PLOS ONE 10 (9): e0136317. doi:10.1371/journal.pone.0136317. ISSN 1932-6203. PMID 26325687. Bibcode: 2015PLoSO..1036317H.
- ↑ Laugel-Haushalter, Virginie; Morkmued, Supawich; Stoetzel, Corinne; Geoffroy, Véronique; Muller, Jean; Boland, Anne; Deleuze, Jean-François; Chennen, Kirsley et al. (2018). "Genetic Evidence Supporting the Role of the Calcium Channel, CACNA1S, in Tooth Cusp and Root Patterning" (in en). Frontiers in Physiology 9: 1329. doi:10.3389/fphys.2018.01329. ISSN 1664-042X. PMID 30319441.
- ↑ Abdelkreem, Elsayed; Akella, Radha Rama Devi; Dave, Usha; Sane, Sudhir; Otsuka, Hiroki; Sasai, Hideo; Aoyama, Yuka; Nakama, Mina et al. (2016-12-08), "Clinical and Mutational Characterizations of Ten Indian Patients with Beta-Ketothiolase Deficiency", JIMD Reports (Springer Berlin Heidelberg) 35: 59–65, doi:10.1007/8904_2016_26, ISBN 9783662558324, PMID 27928777
- ↑ Yıldız Bölükbaşı, Esra; Afzal, Muhammad; Mumtaz, Sara; Ahmad, Nafees; Malik, Sajid; Tolun, Aslıhan (2017-06-21). "Progressive SCAR14 with unclear speech, developmental delay, tremor, and behavioral problems caused by a homozygous deletion of the SPTBN2 pleckstrin homology domain". American Journal of Medical Genetics Part A 173 (9): 2494–2499. doi:10.1002/ajmg.a.38332. ISSN 1552-4825. PMID 28636205.
- ↑ Szczepanska, Maria; Zaniew, Marcin; Recker, Florian; Mizerska-Wasiak, Malgorzata; Zaluska-Lesniewska, Iga; Kilis-Pstrusinska, Katarzyna; Adamczyk, Piotr; Zawadzki, Jan et al. (October 2015). "Dent disease in children: diagnostic and therapeutic considerations". Clinical Nephrology 84 (4): 222–230. doi:10.5414/CN108522. ISSN 0301-0430. PMID 26308078.
- ↑ Noris, Marina; Remuzzi, Giuseppe (2009-10-22). "Atypical Hemolytic–Uremic Syndrome". New England Journal of Medicine 361 (17): 1676–1687. doi:10.1056/nejmra0902814. ISSN 0028-4793. PMID 19846853.
- ↑ Genetics of age-related macular degeneration and Stargardt disease in South African populations. Ramesar, Rajkumar, Roberts, Lisa. 2016. https://open.uct.ac.za/handle/11427/20328.
- ↑ 73.0 73.1 73.2 73.3 73.4 73.5 73.6 Shapiro, M B; Senapathy, P (1987-09-11). "RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression.". Nucleic Acids Research 15 (17): 7155–7174. doi:10.1093/nar/15.17.7155. ISSN 0305-1048. PMID 3658675.
- ↑ Spirio, L.; Olschwang, S.; Groden, J.; Robertson, M.; Samowitz, W.; Joslyn, G.; Gelbert, L.; Thliveris, A. et al. (1993-12-03). "Alleles of the APC gene: an attenuated form of familial polyposis". Cell 75 (5): 951–957. doi:10.1016/0092-8674(93)90538-2. ISSN 0092-8674. PMID 8252630.
- ↑ Davoodi‐Semiromi, Abdoreza; Lanyon, George W.; Davidson, Rosemary; Connor, Michael J. (2000-11-06). "Aberrant RNA splicing in the hMSH2 gene: Molecular identification of three aberrant RNA in Scottish patients with colorectal cancer in the West of Scotland". American Journal of Medical Genetics 95 (1): 49–52. doi:10.1002/1096-8628(20001106)95:1<49::aid-ajmg10>3.0.co;2-p. ISSN 1096-8628. PMID 11074494.
- ↑ Whittock, Neil Vincent; Sher, Carron; Gold, Isaac; Libman, Vitalia; Reish, Orit (November 2011). "A founder COL17A1 splice site mutation leading to generalized atrophic benign epidermolysis bullosa in an extended inbred Palestinian family from Israel". Genetics in Medicine 5 (6): 435–439. doi:10.1097/01.gim.0000096494.61125.d8. ISSN 1098-3600. PMID 14614394.
- ↑ van den Hurk, José A. J. M.; van de Pol, Dorien J. R.; Wissinger, Bernd; van Driel, Marc A.; Hoefsloot, Lies H.; de Wijs, Ilse J.; van den Born, L. Ingeborgh; Heckenlively, John R. et al. (2003-06-25). "Novel types of mutation in the choroideremia (CHM) gene: a full-length L1 insertion and an intronic mutation activating a cryptic exon". Human Genetics 113 (3): 268–275. doi:10.1007/s00439-003-0970-0. ISSN 0340-6717. PMID 12827496.
- ↑ Kesarwani, A K; Ramirez, O; Gupta, A K; Yang, X; Murthy, T; Minella, A C; Pillai, M M (2016-08-15). "Cancer-associated SF3B1 mutants recognize otherwise inaccessible cryptic 3′ splice sites within RNA secondary structures". Oncogene 36 (8): 1123–1133. doi:10.1038/onc.2016.279. ISSN 0950-9232. PMID 27524419.
- ↑ Infante, Joana B.; Alvelos, Maria I.; Bastos, Margarida; Carrilho, Francisco; Lemos, Manuel C. (January 2016). "Complete androgen insensitivity syndrome caused by a novel splice donor site mutation and activation of a cryptic splice donor site in the androgen receptor gene". The Journal of Steroid Biochemistry and Molecular Biology 155 (Pt A): 63–66. doi:10.1016/j.jsbmb.2015.09.042. ISSN 0960-0760. PMID 26435450.
- ↑ Niba, E.; Nishuda, A.; Tran, V.; Vu, D.; Matsumoto, M.; Awano, H.; Lee, T.; Takeshima, Y. et al. (June 2016). "Cryptic splice site activation by a splice donor site mutation of dystrophin intron 64 is determined by intronic splicing regulatory elements". Neuromuscular Disorders 26: S96. doi:10.1016/j.nmd.2016.06.042. ISSN 0960-8966.
- ↑ Salas, Pilar Carrasco; Rosales, José Miguel Lezana; Milla, Carmen Palma; Montiel, Javier López; Siles, Juan López (2015-08-27). "A novel mutation in the β-spectrin gene causes the activation of a cryptic 5′-splice site and the creation of a de novo 3′-splice site". Human Genome Variation 2 (1): 15029. doi:10.1038/hgv.2015.29. ISSN 2054-345X. PMID 27081538.
- ↑ Qadah, Talal; Finlayson, Jill; Joly, Philippe; Ghassemifar, Reza (2013-11-25). "Molecular and Cellular Analysis of a NovelHBA2Mutation (HBA2: c.94A>G) Shows Activation of a Cryptic Splice Site and Generation of a Premature Termination Codon". Hemoglobin 38 (1): 13–18. doi:10.3109/03630269.2013.858639. ISSN 0363-0269. PMID 24274170.
- ↑ Shi, Xiao-Xiao; Huang, Yuan-Jie; Begum, Mahfuj-Ara; Zhu, Mu-Fei; Li, Fei-Qiang; Zhang, Min-Jing; Zhou, Wen-Wu; Mao, Cungui et al. (2018-01-18). "A neutral ceramidase, NlnCDase, is involved in the stress responses of brown planthopper, Nilaparvata lugens (Stål)". Scientific Reports 8 (1): 1130. doi:10.1038/s41598-018-19219-y. ISSN 2045-2322. PMID 29348442. Bibcode: 2018NatSR...8.1130S.
- ↑ 84.0 84.1 Gasparini, Fabio; Skobo, Tatjana; Benato, Francesca; Gioacchini, Giorgia; Voskoboynik, Ayelet; Carnevali, Oliana; Manni, Lucia; Valle, Luisa Dalla (2016-02-01). "Characterization of Ambra1 in asexual cycle of a non-vertebrate chordate, the colonial tunicate Botryllus schlosseri, and phylogenetic analysis of the protein group in Bilateria". Molecular Phylogenetics and Evolution 95: 46–57. doi:10.1016/j.ympev.2015.11.001. ISSN 1055-7903. PMID 26611831.
- ↑ 85.0 85.1 Maragh, Samantha; Miller, Ronald A.; Bessling, Seneca L.; Wang, Guangliang; Hook, Paul W.; McCallion, Andrew S. (2014-08-29). "Rbm24a and Rbm24b Are Required for Normal Somitogenesis". PLOS ONE 9 (8): e105460. doi:10.1371/journal.pone.0105460. ISSN 1932-6203. PMID 25170925. Bibcode: 2014PLoSO...9j5460M.
- ↑ 86.0 86.1 Juliant, Sylvie; Harduin-Lepers, Anne; Monjaret, François; Catieau, Béatrice; Violet, Marie-Luce; Cérutti, Pierre; Ozil, Annick; Duonor-Cérutti, Martine (2014-10-21). "The α1,6-Fucosyltransferase Gene (fut8) from the Sf9 Lepidopteran Insect Cell Line: Insights into fut8 Evolution". PLOS ONE 9 (10): e110422. doi:10.1371/journal.pone.0110422. ISSN 1932-6203. PMID 25333276. Bibcode: 2014PLoSO...9k0422J.
- ↑ Hooper, John D.; Campagnolo, Luisa; Goodarzi, Goodarz; Truong, Tony N.; Stuhlmann, Heidi; Quigley, James P. (2003-08-01). "Mouse matriptase-2: identification, characterization and comparative mRNA expression analysis with mouse hepsin in adult and embryonic tissues". Biochemical Journal 373 (3): 689–702. doi:10.1042/bj20030390. ISSN 0264-6021. PMID 12744720.
- ↑ 88.0 88.1 Xue, Chenxiao; Zhang, Huawei; Lin, Qiupeng; Fan, Rong; Gao, Caixia (2018-09-27). "Manipulating mRNA splicing by base editing in plants". Science China Life Sciences 61 (11): 1293–1300. doi:10.1007/s11427-018-9392-7. ISSN 1674-7305. PMID 30267262.
- ↑ 89.0 89.1 Michalko, Jaroslav; Renner, Tanya; Mészáros, Patrik; Socha, Peter; Moravčíková, Jana; Blehová, Alžbeta; Libantová, Jana; Polóniová, Zuzana et al. (2016-08-31). "Molecular characterization and evolution of carnivorous sundew (Drosera rotundifolia L.) class V β-1,3-glucanase". Planta 245 (1): 77–91. doi:10.1007/s00425-016-2592-5. ISSN 0032-0935. PMID 27580619.
- ↑ 90.0 90.1 Wongkantrakorn, N.; Duangsrisai, S. (2015-02-15). "The level of mRNA NAD-SDH is regulated through RNA splicing by sugars and phytohormones". Russian Journal of Plant Physiology 62 (2): 279–282. doi:10.1134/s1021443715010161. ISSN 1021-4437.
- ↑ Feng, Jiayue; Li, Jing; Liu, Hong; Gao, Qinghua; Duan, Ke; Zou, Zhirong (2012-10-03). "Isolation and Characterization of a Calcium-Dependent Protein Kinase Gene, FvCDPK1, Responsive to Abiotic Stress in Woodland Strawberry (Fragaria vesca)". Plant Molecular Biology Reporter 31 (2): 443–456. doi:10.1007/s11105-012-0513-8. ISSN 0735-9640.
- ↑ Philip, Anna; Syamaladevi, Divya P.; Chakravarthi, M.; Gopinath, K.; Subramonian, N. (2013-03-19). "5′ Regulatory region of ubiquitin 2 gene from Porteresia coarctata makes efficient promoters for transgene expression in monocots and dicots". Plant Cell Reports 32 (8): 1199–1210. doi:10.1007/s00299-013-1416-3. ISSN 0721-7714. PMID 23508257.
- ↑ 93.0 93.1 93.2 Senapathy, P (April 1986). "Origin of eukaryotic introns: a hypothesis, based on codon distribution statistics in genes, and its implications.". Proceedings of the National Academy of Sciences of the United States of America 83 (7): 2133–2137. doi:10.1073/pnas.83.7.2133. ISSN 0027-8424. PMID 3457379. Bibcode: 1986PNAS...83.2133S.
- ↑ 94.0 94.1 94.2 94.3 Senapathy, P (February 1988). "Possible evolution of splice-junction signals in eukaryotic genes from stop codons.". Proceedings of the National Academy of Sciences of the United States of America 85 (4): 1129–1133. doi:10.1073/pnas.85.4.1129. ISSN 0027-8424. PMID 3422483. Bibcode: 1988PNAS...85.1129S.
- ↑ Information, Reed Business (1986-06-26). New Scientist. Reed Business Information. https://books.google.com/books?id=oZjRIhZtINUC&pg=PA34.
- ↑ Information, Reed Business (1988-03-31). New Scientist. Reed Business Information. https://books.google.com/books?id=yGdGbzlA6AQC&pg=PA31.
- ↑ "Revisiting the Five Splice Site Algorithms used in Clinical Genetics". Our 2 SNPs...®. 2018-04-26. http://blog.goldenhelix.com/nfortier/clinical-variant-interpretation-part-v/.
- ↑ Bhasi, Ashwini; Pandey, Ram Vinay; Utharasamy, Suriya Prabha; Senapathy, Periannan (2007-03-07). "EuSplice: a unified resource for the analysis of splice signals and alternative splicing in eukaryotic genes". Bioinformatics 23 (14): 1815–1823. doi:10.1093/bioinformatics/btm084. ISSN 1460-2059. PMID 17344236.
- ↑ Bhasi, Ashwini; Philip, Philge; Sreedharan, Vipin T.; Senapathy, Periannan (July 2009). "AspAlt: A tool for inter-database, inter-genomic and user-specific comparative analysis of alternative transcription and alternative splicing in 46 eukaryotes". Genomics 94 (1): 48–54. doi:10.1016/j.ygeno.2009.02.006. ISSN 0888-7543. PMID 19285128.
- ↑ Bhasi, Ashwini; Philip, Philge; Manikandan, Vinu; Senapathy, Periannan (2008-11-04). "ExDom: an integrated database for comparative analysis of the exon–intron structures of protein domains in eukaryotes". Nucleic Acids Research 37 (suppl_1): D703–D711. doi:10.1093/nar/gkn746. ISSN 1362-4962. PMID 18984624.
- ↑ Bhasi, Ashwini; Senalik, Doug; Simon, Philipp W; Kumar, Brajendra; Manikandan, Vinu; Philip, Philge; Senapathy, Periannan (2010). "RoBuST: an integrated genomics resource for the root and bulb crop families Apiaceae and Alliaceae". BMC Plant Biology 10 (1): 161. doi:10.1186/1471-2229-10-161. ISSN 1471-2229. PMID 20691054.
- ↑ Dorman, Nijsje (June 2009). "Citations". BioTechniques 46 (7): 495. doi:10.2144/000113175. ISSN 0736-6205.
