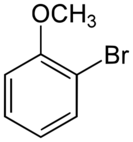
Chemistry:2-Bromoanisole
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Bromo-2-methoxybenzene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| Appearance | colorless liquid |
| Melting point | 2.5 °C (36.5 °F; 275.6 K) |
| Boiling point | 216 °C (421 °F; 489 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H411 | |
| P273, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2-Bromoanisole is an organobromide with the formula BrC6H4OCH3. A colorless liquid, it is one of three isomers of bromoanisole, the others being 3-bromoanisole and 4-bromoanisole. It is a standard coupling partner in metal catalyzed coupling reactions. These reactions include Heck reactions, Buchwald-Hartwig coupling,[1] Suzuki couplings, and Ullmann condensations.[2] The corresponding Grignard reagent readily forms. It is a precursor to o-anisaldehyde.[3][4]
References
- ↑ Klapars, Artis; Antilla, Jon C.; Huang, Xiaohua; Buchwald, Stephen L. (2001). "A General and Efficient Copper Catalyst for the Amidation of Aryl Halides and theN-Arylation of Nitrogen Heterocycles". Journal of the American Chemical Society 123 (31): 7727–7729. doi:10.1021/ja016226z. PMID 11481007.
- ↑ Buck, Elizabeth; Song, Zhiguo J. (2005). "Preparation of 1-Methoxy-2-(4-Methoxyphenoxy)Benzene". Organic Syntheses 82: 69. doi:10.15227/orgsyn.082.0069.
- ↑ Sisti, A. J. (1964). "O-Anisaldehyde". Organic Syntheses 44: 4. doi:10.15227/orgsyn.044.0004.
- ↑ Brinkmeyer, R. S.; Collington, E. W.; Meyers, A. I. (1974). "Aldehydes from 4,4-Dimethyl-2-oxazoline and Grignard Reagents: O-Anisaldehyde". Organic Syntheses 54: 42. doi:10.15227/orgsyn.054.0042.
 |

