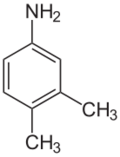
Chemistry:3,4-Xylidine
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dimethylaniline | |
| Other names
3,4-Dimethylphenylamine
3,4-Dimethylbenzenamine | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.183 g·mol−1 |
| Melting point | 51.0 °C (123.8 °F; 324.1 K) |
| Boiling point | 226.0 °C (438.8 °F; 499.1 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3,4-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless solid. It is a precursor for the production of riboflavin (vitamin B2).[1]
The compound is prepared by two routes: hydrogenation of (2-chloromethyl)-4-nitrotoluene and reaction of the bromoxylene with ammonia.[1]
Safety
Like other xylidines, 3,4-xylidine has modest toxicity with an -1">50 of 812 mg/kg when administered orally to rats.[1]
In 2003, more than twenty US Army troops were allegedly exposed to 3,4-xylidine during the occupation of Iraq, leading to a number of health complaints.[2]
References
- ↑ 1.0 1.1 1.2 M. Meyer (2012). "Xylidines". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455. ISBN 978-3527306732.
- ↑ "12 Years Later, a Mystery of Chemical Exposure in Iraq Clears Slightly". New York Times. May 14, 2015. https://www.nytimes.com/2015/05/15/world/middleeast/12-years-later-a-mystery-of-chemical-exposure-in-iraq-clears-slightly.html.
 |

