Chemistry:Cellocidin
From HandWiki
Revision as of 01:11, 8 May 2022 by imported>WikiGary (add)
| |
| Names | |
|---|---|
| Preferred IUPAC name
But-2-ynediamide | |
| Other names
Cellocidin; Lenamycin; Acetylene diamide; Acetylenedicarboxamide; Acetylenedicarboxylic acid diamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| MeSH | C015739 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C4H4N2O2 | |
| Molar mass | 112.088 g·mol−1 |
| Density | 1.411 g/mL |
| Melting point | 179 °C (354 °F; 452 K) |
| log P | -1.2 |
| Hazards | |
| Main hazards | Skin irritant; may cause respiratory tract irritation |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
| Flash point | 216 °C (421 °F; 489 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
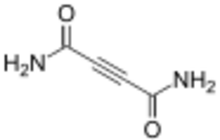
Cellocidin (2-butynediamide) is an organic chemical compound with the molecular formula C4H4O2N2. This compound was isolated from Streptomyces chibaensis, Streptomyces reticuli and Streptomyces sp. SF-536.
Structure
Cellocidin is an organic compound containing 4 carbon atoms, 4 hydrogen atoms, 2 oxygen atoms, and 2 nitrogen atoms. It contains one carbon-carbon triple bond between carbons 2 and 3, and two carbon-oxygen double bonds off carbon 1 and carbon 4. Cellocidin contains two identical amide groups connected together by the central carbons (carbons 2 and 3).
References
- Jones, Ewart R. H.; Keeping, J. W.; Pellatt, M. G.; Thaller, V. (1973). "Natural acetylenes. Part XXXVIII. Biosyntheses of acetylenedicarboxamide (cellocidin) in Streptomyces SF-536 cultures". Journal of the Chemical Society, Perkin Transactions 1 1973 (1): 148–150. doi:10.1039/P19730000148. PMID 4736298.
 |

