Chemistry:Isoborneol
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
(1S,2S,4S)-1,7,7-trimethylbicyclo[2.2.1]heptane-2-ol, (1R,2R,4R)-1,7,7-trimethylbicyclo[2.2.1]heptane-2-ol
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| UN number | 1312 |
| |
| |
| Properties | |
| C10H18O | |
| Molar mass | 154.253 g·mol−1 |
| Appearance | white or colorless solid |
| Melting point | 212–214 °C (414–417 °F; 485–487 K) + or -; 210–215 °C for rac |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H228 | |
| P210, P240, P241, P280, P370+378 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
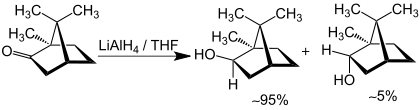
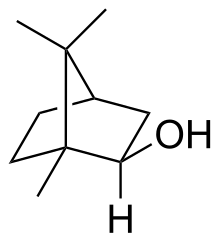
Isoborneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an exo position. The endo diastereomer is called borneol. Being chiral, isoborneol exists as enantiomers.
Preparation
Isoborneol is synthesized commercially by hydrolysis of isobornyl acetate. The latter is obtained from treatment of camphene with acetic acid in the presence of a strong acid catalyst.[2]
It can also be produced by reduction of camphor:
Isoborneol derivatives as chiral ligands
Derivatives of isoborneol are used as ligands in asymmetric synthesis.[3]
- (2S)-(−)-3-exo-(morpholino)isoborneol or MIB[4] with a morpholine substituent in the α-hydroxyl position.
- (2S)-(−)-3-exo-(dimethylamino)isoborneol or DAIB[5] with a dimethylamino substituent in the α-hydroxyl position
References
- ↑ "(+)-Isoborneol" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/6973640#section=Safety-and-Hazards.
- ↑ Sell, Charles S. (2006). "Terpenoids". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.2005181602120504.a01.pub2. ISBN 0471238961.
- ↑ Yus, Miguel; Ramón, Diego (2007). "Chiral Ligands with an Isoborneol-10-sulfonamide Structure: A Ten-Year Odyssey". Synlett 2007 (15): 2309–2320. doi:10.1055/s-2007-985602.
- ↑ Chen, Y. K.; Jeon, S.-J.; Walsh, P. J.; Nugent, W. A. (2005). "(2S)-(−)-3-exo-(Morpholino)isoborneol". Organic Syntheses 82: 87. doi:10.15227/orgsyn.082.0087.
- ↑ White, J. D.; Wardrop, D. J.; Sundermann, K. F. (2002). "(2S)-(−)-3-exo-(Dimethylamino)isoborneol". Organic Syntheses 79: 130. doi:10.15227/orgsyn.079.0130.
 |