Chemistry:Phenidone
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
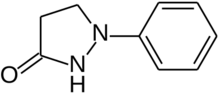
1-Phenylpyrazolidin-3-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H10N2O | |
| Molar mass | 162.192 g·mol−1 |
| Appearance | Crystal leaflets or needles |
| Melting point | 121 °C (250 °F; 394 K) |
| 10 g/100 ml at 100 °C | |
| Solubility in ethanol | 10 g/100 ml (hot) |
| Solubility in diethyl ether | practically insoluble |
| Hazards | |
| Main hazards | Harmful if swallowed |
| Safety data sheet | External MSDS |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H411 | |
| P264, P270, P273, P301+312, P330, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phenidone (1-phenyl-3-pyrazolidinone) is an organic compound that is primarily used as a photographic developer. It has five to ten times the developing power as Metol. It also has low toxicity and unlike some other developers, does not cause dermatitis upon skin contact.[1]
Phenidone is Ilford's trademark for this material, which was first prepared in 1890. It was not until 1940 that J. D. Kendall, in the laboratories of Ilford Limited, discovered the reducing properties of this compound. Large scale production did not become feasible until 1951.[2]
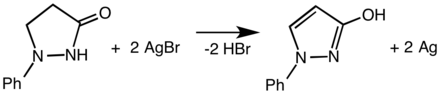
Phenidone functions as a reducing agent. It converts to the N-phenyl-hydroxypyrazole:
Preparation
Phenidone can be prepared by heating phenyl hydrazine with 3-chloropropanoic acid.[1]
References
- ↑ 1.0 1.1 Merck Index of Chemicals and Drugs, 9th ed. monograph 7115
- ↑ Karlheinz Keller et al. "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_001
 |