Chemistry:Nickel(II) precatalysts
Nickel(II) precatalysts are a type of catalyst used in organic reactions. Many transformations are catalyzed by nickel in organometallic chemistry and in organic synthesis.[1] Many of these transformations invoke a low valent (generally Ni(0)) species as the active catalyst. Unfortunately, unlike its counterpart, Pd(0), Ni(0) catalysts are predominantly confined to the glovebox due to their high instability to air and water, with the most common Ni(0) catalyst being Ni(cod)2. Additionally, Ni(cod)2 is more expensive than many Ni(II) salts and the quality varies significantly amongst suppliers.[2] To make nickel catalysis more accessible and amenable to synthesis and industrial purposes, the use of air-stable Ni(II) precursors has emerged as an important development in this area of research. This page describes the more commonly employed nickel(II) precatalysts, their synthesis for those not commercially available, and the methods for their reduction to Ni(0) complexes.
Synthesized Air and Moisture-Stable Nickel Precatalysts
Ni(PPh3)2-(1-naph)Cl
Ni(II) precatalyst promote Suzuki cross coupling reactions between chloroarenes and arylboronic acids.[3] This catalyst had previously been used by Brandsma in his cyanation of thiophenes.[4] Whereas commercially available Ni(II) catalysts such as NiCl2(PPh3)2 and NiCl2(dppe) did not catalyze Yang's reaction, the Ni(II) catalyst Ni(PPh3)2-(1-naph)Cl yielded 24% of the desired product. Further optimization provided the desired products in 87-99% yield at 60 °C. File:Yang catalyst.tif
Activation of the Ni(II) complex is proposed to occur through transmetalation of the Ni(II) precatalyst with the boronic acid results in a biaryl Ni(II) intermediate which undergoes reductive elimination to product the biaryl product and Ni(0).
[(dppf)Ni(cinnamyl)Cl)]
An air and moisture stable Ni(II) precatalyst promotes Suzuki-Miyuara cross-coupling of heteroaryl boronic acids with nitrogen- and sulfur-containing heteroaryl halides.[5] The reactions proceeded with 0.5 mol% of [(dppf)Ni(cinnamyl)Cl)] without the need for added ligand in 81-97% yield. The catalyst is stable under air in a closed vial at 0 °C for at least two weeks.
The authors investigated the reduction of [(dppf)Ni(cinnamyl)Cl)] and found that [(dppf)2Ni(0)] was formed quantitatively in 10 minutes at room temperature upon the addition of 2-thienyl boronic acid and K3PO4.
trans-(PCy2Ph)2Ni(o-tolyl)Cl
Air stable Ni(II) precatalyst have been developed for Mizoroki-Heck reactions.[2] After several attempts to develop an air-stable precatalyst, the group came upon trans-(PCy2Ph)2Ni(o-tolyl)Cl, which catalyzes the coupling of benzyl chloride and terminal alkenes in 41-96% yield. File:Jamison Synthesis.tif
The precatalyst can be reduced to Ni(0) via treatment TMSOTf and base at room temperature within minutes. The authors propose that upon chloride abstraction from the Ni(II) precatalyst, transmetalation between the resulting cationic nickel complex and a second Ni(II) precatalyst produces (PCy2Ph)2Ni(o-tolyl)2 and (PCy2Ph)2Ni(Cl)(OTf). Reductive elimination from (PCy2Ph)2Ni(o-tolyl)2 results in the catalytically active Ni(0) complex. This paper documents the first use of silyl triflate to generate Ni(0). File:Johnson.tif
(dppf)Ni(o-tolyl)Cl
Air-stable Ni(II) precatalysts also promote the amination of aryl chlorides, sulfamates, mesylates, and triflates.[6] The nickel catalyst, (dppf)Ni(o-tolyl)Cl, can be prepared from ligand exchange with (PPh3)2Ni(o-tolyl)Cl at room temperature in THF. The authors found that additive of MeCN was crucial for high yields of the coupled product. File:Buchwald Synthesis.tif
[(TMEDA)Ni(o-tolyl)Cl]
Interest remains in Ni(II) precatalyst that are air and moisture stable, inexpensive to make, modular (in terms of compatibility with multiple ligand classes), and general (active in numerous reactions). The precatalysts listed above all contain phosphine ligands, limiting their generality for use in other reactions. Simultaneously, the Doyle group [7] and a group at Pfizer [8] speculated that use of N,N,N’,N’-tetramethylethylenediamine (TMEDA) as a dummy ligand would allow for in situ ligand exchange with the desired ligand for each particular reaction. The complex [(TMEDA)Ni(o-tolyl)Cl] had the desired properties, showing stability on the bench for at least 3 months. The precatalyst is modular can be used in combination with monodentate and bidentate phosphines, diimine ligands, and NHC ligands. Furthermore, the precatalyst showed comparable results in a wide variety of reactions to other Ni(II) precatalysts or Ni(cod)2.
The reduction of the precatalyst to Ni(0) can occur through several different pathways contingent on the reaction conditions. In reactions containing a boronic acid, the precatalyst can be reduced via transmetalation between the nickel and boron, resulting in the biaryl species which can undergo reductive elimination. In cases where no transmetalating agent or amide ion was present for reduction, a nickel-nickel transmetalation event could occur, giving a biaryl nickel complex and LnNiCl2, which upon reduction of the biaryl, would result in the active Ni(0) complex.
N,N,N-Pincer Complex
Ni(II) N,N,N pincer complexes are active in Kumada, Sonogashira, and Suzuki-Miyaura coupling reactions with unactivated alkyl halides.[9] In 2016, the group further developed the catalyst to allow for a broader range of coupling partners.[10] The catalyst is air stable and made from NiCl2-glyme. While the complex is never formally reduced to low valent nickel, the complex first undergoes transmetalation with a Grignard reagent (in the Kumada coupling) resulting in a LnNi(II)-alkyl species which can undergo subsequent oxidative addition with the coupling partner. File:Hu N,N,N pincer.tif
Commonly used commercially available Ni(II) salts
Commonly, commercially available Ni(II) salts are employed in cross-coupling reactions. These salts require reduction to low-valent nickel to obtain the catalytically active Ni(0) or Ni(I) species. Below is a list of common Ni(II) salts and a representative publication utilizing these salts. Empirically, these precatalysts can be used interchangeably, with the effect on yield ranging from quite minor to loss of reactivity.
NiCl2
While NiCl2 anhydrous is not commonly employed as a precatalyst, the NiCl2-6H2O has seen some use and can be used when cost is a concern. More commonly, NiCl2-dme (or NiCl2-glyme) is used due to its increased solubility in comparison to the hexahydrate.[11] File:NiCl2 schemes.tif
NiBr2
NiBr2-glyme, and NiBr2-diglyme have been commonly employed by Jarvo and Fu among others, with diglyme showing increased solubility. Empirically, NiBr2-glyme has shown increased reactivity compared to that of NiCl2-glyme for some transformations.[12] File:NiBr2 scheme.tif
NiI2
Hydrated nickel iodide has been used.[13] File:NiI2 scheme.tif
Ni(acac)2 and Ni(OAc)2
Ni(OTf)2 and Ni(BF4)2
Dichloridobis(triphenylphosphane)nickel(II)
Ni(dcype)(CO)2
Methods for reduction
Metallic Reductants
Addition of Zn0, Mn0, or sodiumamalgam is commonly seen in combination with these NiX2 salts.[2] A common additive in these cross-coupling reactions is NaI, which some authors have proposed could serve to help promote the electron transfer between Mn and Ni.[18] Weix and coworkers found that by activating the Zn with 2% HCl for 1 minute, reaction times were significantly decreased with little change in the overall yield.[19] Similarly, TMSCl can be used to activate the Mn powder. Both of these techniques remove metal oxides on the surface of the dust, which may inhibit reactivity. Due to the heterogeneity of the reaction mixture, yields are contingent on the rate of stirring, which can pose a problem for larger scale (i.e. process-scale) reactions. Additionally, Weix and coworkers found that reactivity is also contingent on the source and size of the zinc dust.[19]
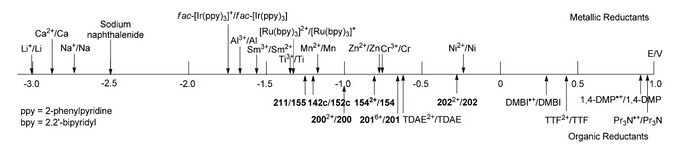
Tetrakis(dimethylamino)ethylene (TDAE) can also function as a single-electron reductant. It can be used to rule out the intermediacy of an in situ formed alkylzinc reagent.[20] Below is a list of redox potentials.[21]
Organometallic
As shown above, many Ni(II) precatalysts can be activated via transmetalation with an organometallic reagent, i.e. boronic acid, organozinc, Grignard. Often this will lead to a LnNiArAr, which can reductive eliminate to give a biaryl compound and LnNi0, and LnNiX2 complex.[2] Alternatively, in the case of Doyle’s catalyst above where two nickel complexes transmetalate, half of the precatalyst is filtered to an inactive LnNiII complex.[7]
Several groups have also invoked Ni(I) as the active nickel catalyst. In these instances, it is possible that an initial transmetalation results in a LnNiIIXR intermediate, which can undergo disproportionation to LnNiX2 and LnNiR2. Subsequent reductive elimination from LnNiR2 results in a Ni(0) intermediate which can disproportionate into LnNiIX.[22]
If the coupling reagent is not an organometallic, a sacrificial organometallic reagent such as AlMe3, Et2Zn, or MeMgBr can be added to the reaction to reduce the Ni(II) to Ni(0). This works through two successive transmetalations, yielding a dialkylnickel(II) species, which will readily undergo reductive elimination to release an alkane and the Ni(0) species.[2]
Hydride Donor
Nickel can readily undergo the formation of a Ni-H in the presence of a hydride source. From a NiX2 precatalyst, two successive transmetalations result in NiH2 which quickly gives off H2 to yield Ni(0). Examples of hydride donors that can effect this transformation are DIBAL, methanol, isopropanol, and various silanes.[2][23]
Photoredox
Nickel has found widespread use in the field of photoredox catalysis. Commonly, Ni(II) salts (ex. NiCl2-glyme) are employed as precatalysts in these reactions and can be reduced via single electron transfer by the photocatalysts (Ir or Ru) to obtain the active Ni(0) catalyst.[24]
References
- ↑ Tasker, Sarah Z.; Standley, Eric A.; Jamison, Timothy F. (2014). "Recent advances in homogeneous nickel catalysis". Nature 509 (7500): 299–309. doi:10.1038/nature13274. PMID 24828188. PMC 4344729. Bibcode: 2014Natur.509..299T. http://dspace.mit.edu/bitstream/1721.1/103919/1/Jamison_Recent%20advances.pdf.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Standley, Eric A.; Jamison, Timothy F. (2013-01-30). "Simplifying Nickel(0) Catalysis: An Air-Stable Nickel Precatalyst for the Internally Selective Benzylation of Terminal Alkenes". Journal of the American Chemical Society 135 (4): 1585–1592. doi:10.1021/ja3116718. PMID 23316879.
- ↑ Chen, Chen; Yang, Lian-Ming (2007-03-26). "Nickel(II)–aryl complexes as catalysts for the Suzuki cross-coupling reaction of chloroarenes and arylboronic acids". Tetrahedron Letters 48 (13): 2427–2430. doi:10.1016/j.tetlet.2007.01.175.
- ↑ Soolingen, J. van; Verkruijsse, H. D.; Keegstra, M. A.; Brandsma, L. (1990-11-01). "Nickel-Catalyzed Cyanation of 2- and 3- Bromothiophene". Synthetic Communications 20 (20): 3153–3156. doi:10.1080/00397919008051539.
- ↑ Ge, Shaozhong; Hartwig, John F. (2012-12-14). "Highly Reactive, Single-Component Nickel Catalyst Precursor for Suzuki–Miyuara Cross-Coupling of Heteroaryl Boronic Acids with Heteroaryl Halides" (in en). Angewandte Chemie International Edition 51 (51): 12837–12841. doi:10.1002/anie.201207428. PMID 23136047.
- ↑ Park, Nathaniel H.; Teverovskiy, Georgiy; Buchwald, Stephen L. (2014-01-03). "Development of an Air-Stable Nickel Precatalyst for the Amination of Aryl Chlorides, Sulfamates, Mesylates, and Triflates". Organic Letters 16 (1): 220–223. doi:10.1021/ol403209k. PMID 24283652.
- ↑ 7.0 7.1 Shields, Jason D.; Gray, Erin E.; Doyle, Abigail G. (2015-05-01). "A Modular, Air-Stable Nickel Precatalyst". Organic Letters 17 (9): 2166–2169. doi:10.1021/acs.orglett.5b00766. PMID 25886092.
- ↑ Magano, Javier; Monfette, Sebastien (2015-04-17). "Development of an Air-Stable, Broadly Applicable Nickel Source for Nickel-Catalyzed Cross-Coupling". ACS Catalysis 5 (5): 3120–3123. doi:10.1021/acscatal.5b00498.
- ↑ Csok, Zsolt; Vechorkin, Oleg; Harkins, Seth B.; Scopelliti, Rosario; Hu, Xile (2008-07-01). "Nickel Complexes of a Pincer NN2 Ligand: Multiple Carbon−Chloride Activation of CH2Cl2 and CHCl3 Leads to Selective Carbon−Carbon Bond Formation". Journal of the American Chemical Society 130 (26): 8156–8157. doi:10.1021/ja8025938. PMID 18528995.
- ↑ Di Franco, Thomas; Stojanovic, Marko; Keller, Sébastien Carlos; Scopelliti, Rosario; Hu, Xile (2016-11-01). "A Structure–Activity Study of Nickel NNN Pincer Complexes for Alkyl-Alkyl Kumada and Suzuki–Miyaura Coupling Reactions" (in en). Helvetica Chimica Acta 99 (11): 830–847. doi:10.1002/hlca.201600165. http://infoscience.epfl.ch/record/225084.
- ↑ Cornella, Josep; Edwards, Jacob T.; Qin, Tian; Kawamura, Shuhei; Wang, Jie; Pan, Chung-Mao; Gianatassio, Ryan; Schmidt, Michael et al. (2016-02-24). "Practical Ni-Catalyzed Aryl–Alkyl Cross-Coupling of Secondary Redox-Active Esters". Journal of the American Chemical Society 138 (7): 2174–2177. doi:10.1021/jacs.6b00250. PMID 26835704.
- ↑ Konev, Mikhail O.; Hanna, Luke E.; Jarvo, Elizabeth R. (2016-06-01). "Intra- and Intermolecular Nickel-Catalyzed Reductive Cross-Electrophile Coupling Reactions of Benzylic Esters with Aryl Halides" (in en). Angewandte Chemie International Edition 55 (23): 6730–6733. doi:10.1002/anie.201601206. PMID 27099968.
- ↑ Everson, Daniel A.; Shrestha, Ruja; Weix, Daniel J. (2010-01-27). "Nickel-Catalyzed Reductive Cross-Coupling of Aryl Halides with Alkyl Halides". Journal of the American Chemical Society 132 (3): 920–921. doi:10.1021/ja9093956. PMID 20047282.
- ↑ Shrestha, Ruja; Dorn, Stephanie C. M.; Weix, Daniel J. (2013-01-16). "Nickel-Catalyzed Reductive Conjugate Addition to Enones via Allylnickel Intermediates". Journal of the American Chemical Society 135 (2): 751–762. doi:10.1021/ja309176h. PMID 23270480.
- ↑ Aihara, Yoshinori; Chatani, Naoto (2013-04-10). "Nickel-Catalyzed Direct Alkylation of C–H Bonds in Benzamides and Acrylamides with Functionalized Alkyl Halides via Bidentate-Chelation Assistance". Journal of the American Chemical Society 135 (14): 5308–5311. doi:10.1021/ja401344e. PMID 23495861.
- ↑ Johnson, Jeffrey S.; Berman, Ashley M. (2005-07-01). "Nickel-Catalyzed Electrophilic Amination of Organozinc Halides" (in en). Synlett 2005 (11): 1799–1801. doi:10.1055/s-2005-871567.
- ↑ Muto, Kei; Yamaguchi, Junichiro; Itami, Kenichiro (2012-01-11). "Nickel-Catalyzed C–H/C–O Coupling of Azoles with Phenol Derivatives". Journal of the American Chemical Society 134 (1): 169–172. doi:10.1021/ja210249h. PMID 22148419.
- ↑ Cherney, Alan H.; Reisman, Sarah E. (2014-10-15). "Nickel-Catalyzed Asymmetric Reductive Cross-Coupling Between Vinyl and Benzyl Electrophiles". Journal of the American Chemical Society 136 (41): 14365–14368. doi:10.1021/ja508067c. PMID 25245492.
- ↑ 19.0 19.1 Everson, Daniel A.; Jones, Brittany A.; Weix, Daniel J. (2012-04-11). "Replacing Conventional Carbon Nucleophiles with Electrophiles: Nickel-Catalyzed Reductive Alkylation of Aryl Bromides and Chlorides". Journal of the American Chemical Society 134 (14): 6146–6159. doi:10.1021/ja301769r. PMID 22463689.
- ↑ Anka-Lufford, Lukiana L.; Huihui, Kierra M. M.; Gower, Nicholas J.; Ackerman, Laura K. G.; Weix, Daniel J. (2016-08-08). "Nickel-Catalyzed Cross-Electrophile Coupling with Organic Reductants in Non-Amide Solvents" (in en). Chemistry – A European Journal 22 (33): 11564–11567. doi:10.1002/chem.201602668. PMID 27273457.
- ↑ Broggi, Julie; Terme, Thierry; Vanelle, Patrice (2014-01-07). "Organic Electron Donors as Powerful Single-Electron Reducing Agents in Organic Synthesis" (in en). Angewandte Chemie International Edition 53 (2): 384–413. doi:10.1002/anie.201209060. PMID 24273111. https://hal.archives-ouvertes.fr/hal-01428063/file/Review%20Organic%20Electron%20Donors%20ACIE%202014.pdf.
- ↑ Yamamoto, Takakazu; Wakabayashi, Shoichiro; Osakada, Kohtaro (April 1992). "Mechanism of C–C coupling reactions of aromatic halides, promoted by Ni(COD)2 in the presence of 2,2′-bipyridine and PPh3, to give biaryls". Journal of Organometallic Chemistry 428 (1–2): 223–237. doi:10.1016/0022-328X(92)83232-7.
- ↑ 1964-, Hartwig, John Frederick (2010-01-01). Organotransition metal chemistry : from bonding to catalysis. University Science Books. ISBN 978-1891389535. OCLC 781082054.
- ↑ Zhang, Patricia; Le, Chi “Chip”; MacMillan, David W. C. (2016-07-06). "Silyl Radical Activation of Alkyl Halides in Metallaphotoredox Catalysis: A Unique Pathway for Cross-Electrophile Coupling". Journal of the American Chemical Society 138 (26): 8084–8087. doi:10.1021/jacs.6b04818. PMID 27263662.