Chemistry:Bis(cyclooctadiene)nickel(0)
| |
| Names | |
|---|---|
| Other names
nickel biscod, Ni(COD)2
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 1325 |
| |
| |
| Properties | |
| C16H24Ni | |
| Molar mass | 275.06 g/mol |
| Appearance | Yellow solid |
| Melting point | 60 °C (140 °F; 333 K) (N2, decomposes) |
| Solubility | soluble in benzene, tetrahydrofuran, toluene, diethyl ether, dimethylformamide |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H228, H317, H334, H350, H351 | |
| P201, P202, P210, P240, P241, P261, P272, P280, P281, P285, P302+352, P304+341, P308+313, P321, P333+313, P342+311, P363, P370+378, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
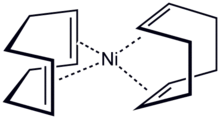
Bis(cyclooctadiene)nickel(0) is the organonickel compound with the formula Ni(C8H12)2, also written Ni(cod)2. It is a diamagnetic coordination complex featuring tetrahedral nickel(0) bound to the alkene groups in two 1,5-cyclooctadiene ligands. This highly air-sensitive yellow solid is a common source of Ni(0) in chemical synthesis.[1]
Preparation and properties
The complex is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin:
- Ni(acac)2 + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4
Ni(cod)2 is moderately soluble in several organic solvents.[2][3] One or both 1,5-cyclooctadiene ligands are readily displaced by phosphines, phosphites, bipyridine, and isocyanides. If exposed to air, the solid oxidizes to nickel(II) oxide.[4] As a result, this compound is generally handled in a glovebox.[5]
References
- ↑ Wilke, G. (1988). "Contributions to Organo-Nickel Chemistry". Angewandte Chemie International Edition 27 (1): 185–206. doi:10.1002/anie.198801851.
- ↑ Schunn, R. A.; Ittel, S. D.; Cushing, M. A. (1990). Bis(1,5-Cyclooctadiene)Nickel(0). Inorganic Syntheses. 28. pp. 94–98. doi:10.1002/9780470132593.ch25. ISBN 978-0-470-13259-3.
- ↑ Wender, Paul A.; Smith, Thomas E.; Duong, Hung A.; Louie, Janis; Standley, Eric A.; Tasker, Sarah Z. (2015). "Bis(1,5-cyclooctadiene)nickel(0)" (in en). Encyclopedia of Reagents for Organic Synthesis (John Wiley & Sons Ltd): 1–15. doi:10.1002/047084289x.rb118.pub3. ISBN 9780470842898.
- ↑ Zhu, Kake; D'Souza, Lawrence; Richards, Ryan M. (September 2005). "Planting of bis(1,5-cyclooctadiene) nickel upon silica to harvest NiO (<5 nm) nanoparticles in a silica matrix". Applied Organometallic Chemistry 19 (9): 1065–1069. doi:10.1002/aoc.974.
- ↑ Tasker, Sarah Z.; Standley, Eric A.; Jamison, Timothy F. (2014). "Recent advances in homogeneous nickel catalysis". Nature 509 (7500): 299–309. doi:10.1038/nature13274. PMID 24828188.
 |

