Chemistry:Kostanecki acylation
From HandWiki
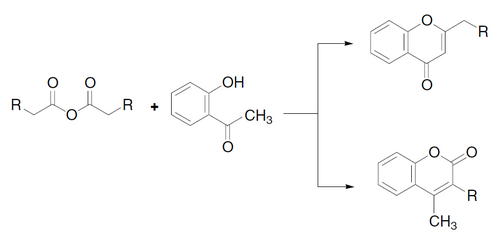
The Kostanecki acylation is a method used in organic synthesis to form chromones[1] or coumarins[2] by acylation of O-hydroxyaryl ketones with aliphatic acid anhydrides, followed by cyclization.[3] If benzoic anhydride (or benzoyl chloride) is used, a particular type of chromone called a flavone is obtained.
Mechanism
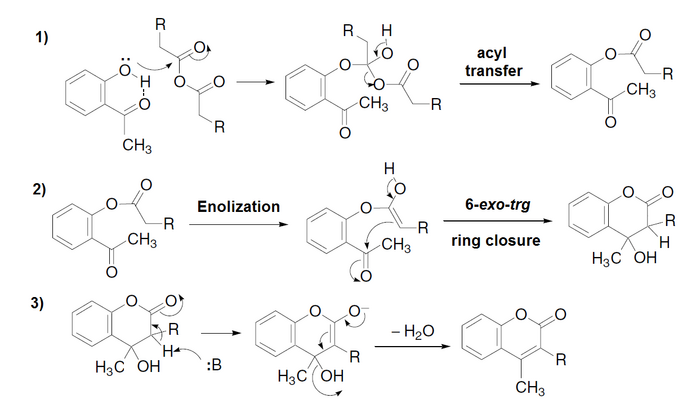
The mechanism consists of three well-differentiated reactions:[4][5]
- Phenol O-acylation with formation of a tetrahedral intermediate
- Intramolecular aldol condensation to cyclize and to form a hydroxydihydrochromone
- Elimination of the hydroxyl group to form the chromone (or coumarin)
Examples
See also
References
- ↑ Gaspar, A.; Matos, M. J. O.; Garrido, J.; Uriarte, E.; Borges, F. (2014). "Chromone: A Valid Scaffold in Medicinal Chemistry". Chemical Reviews 114 (9): 4960–92. doi:10.1021/cr400265z. PMID 24555663. https://repositorio-aberto.up.pt/handle/10216/70808.
- ↑ Sethna, S. M.; Shah, N. M. (1945). "The Chemistry of Coumarins". Chemical Reviews 36: 1–62. doi:10.1021/cr60113a001.
- ↑ v. Kostanecki, St.; Różycki, A. (1901). "Ueber eine Bildungsweise von Chromonderivaten". Berichte der Deutschen Chemischen Gesellschaft 34: 102–109. doi:10.1002/cber.19010340119. https://zenodo.org/record/1426000.
- ↑ Mamedov, V. A.; Kalinin, A. A.; Gubaidullin, A. T.; Litvinov, I. A.; Levin, Ya. A. (2003). "3-Benzoylquinoxalin-2(1H)-one in the Kostanecki-Robinson Reaction. Synthesis and Structure of 2-Oxo-4-phenylpyrano[2,3-bquinoxaline"]. Chemistry of Heterocyclic Compounds 39 (1): 96–100. doi:10.1023/A:1023028927007. http://link.springer.com/10.1023/A:1023028927007.
- ↑ Ellis, G. P. (1977) Chromenes, Chromanones, and Chromones from The Chemistry of Heterocyclic Compounds, Weissberger, A. and Taylor, E. C., eds.; Wiley & Sons: New York, vol. 31, p. 495.
 |