Chemistry:Alvocidib
| |
| |
| Names | |
|---|---|
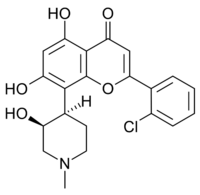
| IUPAC name
2′-Chloro-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]flavone
| |
| Systematic IUPAC name
2-(2-Chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]-4H-1-benzopyran-4-one | |
| Other names
Flavopiridol, HMR 1275, L-868275
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| KEGG | |
| MeSH | Flavopiridol |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H20ClNO5 | |
| Molar mass | 401.8402 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alvocidib (INN; also known as flavopiridol) is a flavonoid alkaloid CDK9 kinase inhibitor under clinical development by Tolero Pharmaceuticals for the treatment of acute myeloid leukemia. It has been studied also for the treatment of arthritis[1] and atherosclerotic plaque formation.[2] The target of alvocidib is the positive transcription elongation factor P-TEFb.[3][4] Treatment of cells with alvocidib leads to inhibition of P-TEFb and the loss of mRNA production.[5][6]
The compound is a synthetic analog of natural product rohitukine which was initially extracted from Aphanamixis polystachya (formerly Amoora rohituka, hence the name) and later from Dysoxylum binectariferum.[7][8]
Orphan drug
The FDA has granted orphan drug designation to alvocidib for the treatment of patients with acute myeloid leukemia.[9]
References
- ↑ "Successful treatment of animal models of rheumatoid arthritis with small-molecule cyclin-dependent kinase inhibitors". J. Immunol. 180 (3): 1954–61. 2008. doi:10.4049/jimmunol.180.3.1954. PMID 18209094.
- ↑ "Flavopiridol inhibits smooth muscle cell proliferation in vitro and neointimal formation In vivo after carotid injury in the rat". Circulation 100 (6): 659–65. 1999. doi:10.1161/01.cir.100.6.659. PMID 10441105.
- ↑ "Flavopiridol inhibits P-TEFb and blocks HIV-1 replication". J. Biol. Chem. 275 (37): 28345–8. 2000. doi:10.1074/jbc.C000446200. PMID 10906320.
- ↑ "Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo". J. Biol. Chem. 276 (34): 31793–9. 2001. doi:10.1074/jbc.M102306200. PMID 11431468.
- ↑ "Functional association of Gdown1 with RNA polymerase II poised on human genes". Mol. Cell 45 (1): 38–50. 2012. doi:10.1016/j.molcel.2011.10.022. PMID 22244331.
- ↑ "c-Myc regulates transcriptional pause release". Cell 141 (3): 432–45. 2010. doi:10.1016/j.cell.2010.03.030. PMID 20434984.
- ↑ Harmon, AD; Weiss, U; Silverton, JV (1979). "The structure of rohitukine, the main alkaloid of Amoora rohituka (syn.Aphanamixis polystachya) (Meliaceae)". Tetrahedron Lett. 20 (1): 721–724. doi:10.1016/S0040-4039(01)93556-7.
- ↑ Lakdawala, AD; Shirole, MV; Mandrekar, SS; Dohadwalla, AN (1988). "Immunopharmacological potential of rohitukine: a novel compound isolated from the plant Dysoxylum binectariferum". Asia Pac J Pharmcol. 3 (1): 91–98.
- ↑ "FDA grants orphan drug status to Alvocidib for AML". http://www.healio.com/hematology-oncology/hematologic-malignancies/news/online/%7B74c6a69e-4529-400d-98e9-d5ee6c602122%7D/fda-grants-orphan-drug-status-to-alvocidib-for-aml.
 |

