Chemistry:Benzoyl chloride
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Benzoyl chloride | |||
| Other names
Benzoic acid chloride (1:1)
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1736 | ||
| |||
| |||
| Properties | |||
| C7H5ClO | |||
| Molar mass | 140.57 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Odor | Benzaldehyde like but more pungent | ||
| Density | 1.21 g/mL, liquid | ||
| Melting point | −1 °C (30 °F; 272 K) | ||
| Boiling point | 197.2 °C (387.0 °F; 470.3 K) | ||
| reacts, forms hydrogen chloride on contact with water | |||
| -75.8·10−6 cm3/mol | |||
| Hazards | |||
| Main hazards | Maybe harmful by ingestion and skin absorption; possible carcinogen[1] | ||
| Safety data sheet | Fisher Scientific MSDS | ||
| GHS pictograms |  
| ||
| GHS Signal word | Danger | ||
| H302, H312, H314, H317, H332 | |||
| P260, P261, P264, P270, P271, P272, P280, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321, P322, P330, P333+313, P363, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 72 °C (162 °F; 345 K) | ||
| Related compounds | |||
Related compounds
|
benzoic acid, benzoic anhydride, benzaldehyde | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
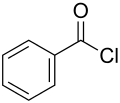
Benzoyl chloride, also known as benzenecarbonyl chloride, is an organochlorine compound with the formula C
7H
5ClO. It is a colourless, fuming liquid with an irritating odour, and consists of a benzene ring (C
6H
6) with an acyl chloride (–C(=O)Cl) substituent. It is mainly useful for the production of peroxides but is generally useful in other areas such as in the preparation of dyes, perfumes, pharmaceuticals, and resins.
Preparation
Benzoyl chloride is produced from benzotrichloride using either water or benzoic acid:[2]
- C
6H
5CCl
3 + H
2O → C
6H
5COCl + 2 HCl - C
6H
5CCl
3 + C
6H
5CO
2H → 2 C
6H
5COCl + HCl
As with other acyl chlorides, it can be generated from the parent acid and standard chlorinating agents such as phosphorus pentachloride, thionyl chloride, and oxalyl chloride. It was first prepared by treatment of benzaldehyde with chlorine.[3]
An early method for production of benzoyl chloride involved chlorination of benzyl alcohol.[4]
Reactions
It reacts with water to produce hydrochloric acid and benzoic acid:
- C
6H
5COCl + H
2O → C
6H
5COOH + HCl
Benzoyl chloride is a typical acyl chloride. It reacts with alcohols to give the corresponding esters. Similarly, it reacts with amines to give the amide.[5][6]
It undergoes the Friedel-Crafts acylation with aromatic compounds to give the corresponding benzophenones and related derivatives.[7] With carbanions, it serves again as a source of the benzoyl cation synthon, C
6H
5CO+
.[8]
Benzoyl peroxide, a common reagent in polymer chemistry, is produced industrially by treating benzoyl chloride with hydrogen peroxide and sodium hydroxide:[9]
- 2 C
6H
5COCl + H
2O
2 + 2 NaOH → (C
6H
5CO)
2O
2 + 2 NaCl + 2 H
2O
References
- ↑ Benzoyl chloride: toxicity and precautions
- ↑ Maki, Takao; Takeda, Kazuo (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555.
- ↑ Friedrich Wöhler, Justus von Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Pharmacie 3 (3): 262–266. doi:10.1002/jlac.18320030302.
- ↑ US1851832, 29 March 1932
- ↑ Marvel, C. S.; Lazier, W. A. (1929). "Benzoyl Piperidine". Organic Syntheses 9: 16. doi:10.15227/orgsyn.009.0016.
- ↑ Prasenjit Saha, Md Ashif Ali, and Tharmalingam Punniyamurthy "Ligand-free Copper(ii) Oxide Nanoparticles Catalyzed Synthesis Of Substituted Benzoxazoles" Org. Synth. 2011, volume 88, pp. 398. doi:10.15227/orgsyn.088.0398. (an illustrative reaction of an amine with benzoyl chloride).
- ↑ Minnis, Wesley (1932). "Phenyl Thienyl Ketone". Organic Syntheses 12: 62. doi:10.15227/orgsyn.012.0062.
- ↑ Fujita, M.; Hiyama, T. (1990). "Directed Reduction of a beta-keto Amide: Erythro-1-(3-hydroxy-2-methyl-3-phenylpropanoyl)piperidine". Organic Syntheses 69: 44. doi:10.15227/orgsyn.069.0044.
- ↑ Template:Cite tech report
External links
 |