Chemistry:Sulfur dicyanide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2N2S | |
| Molar mass | 84.10 g·mol−1 |
| Appearance | white solid |
| Density | 1.48 g/cm3 |
| Melting point | 63.5 °C (146.3 °F; 336.6 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
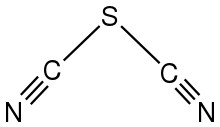
Sulfur dicyanide is an inorganic compound with the formula S(CN)2. A white solid, the compound is mainly of theoretical and fundamental interest given its simplicity. It is the first member of the dicyanosulfanes Sx(CN)2, which includes thiocyanogen ((SCN)2) and higher polysulfanes up to S4(CN)2.[1] According to X-ray crystallography, the molecule is planar, the SCN units are linear, with an S-C-S angle of 95.6°.[2] The synthesis of S(CN)2 is attributed to Söderbäck through his investigation of the reactions of metal cyanides and sulfur halides.[3]
References
- ↑ Steudel, Ralf; Bergemann, Klaus; Kustos, Monika (1994). "Crystal and Molecular Structure of Dicyanotetrasulfane S4(CN)2". Zeitschrift für anorganische und allgemeine Chemie 620: 117–120. doi:10.1002/zaac.19946200119.
- ↑ Emerson, K. (1966). "The Crystal and Molecular Structure of Sulfur Dicyanide". Acta Crystallographica 21 (6): 970–974. doi:10.1107/S0365110X66004262.
- ↑ Söderbäck, Erik (1919). "Studien über das freie Rhodan". Justus Liebig's Annalen der Chemie 419 (3): 217–322. doi:10.1002/jlac.19194190302. https://zenodo.org/record/1427649.
 |

