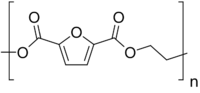
Chemistry:Polyethylene 2,5-furandicarboxylate
| |
| Names | |
|---|---|
| Other names
Polyethylene furanoate; Polyethylene furandicarboxylate; Poly(ethylene furanoate)
| |
| Identifiers | |
| Properties | |
| (C8H6O5)n | |
| Molar mass | Variable |
| Density | 1.43 g/cm3[1] |
| Melting point | 195–265 °C (383–509 °F; 468–538 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polyethylene 2,5-furandicarboxylate, also named poly(ethylene 2,5-furandicarboxylate), polyethylene furanoate and poly(ethylene furanoate) and generally abbreviated as PEF, is a polymer that can be produced by polycondensation or ring-opening polymerization of 2,5-furandicarboxylic acid (FDCA) and ethylene glycol.[2][3] As an aromatic polyester from ethylene glycol it is a chemical analogue of polyethylene terephthalate (PET) and polyethylene naphthalate (PEN). PEF has been described in (patent) literature since 1951,[4] but has gained renewed attention since the US department of energy proclaimed its building block, FDCA, as a potential bio-based replacement for purified terephthalic acid (PTA) in 2004.[5]
Benefits over PET
One life-cycle assessment showed that replacing PTA in the production of PET by bio-based FDCA for the production of PEF has a potential for significant reductions in greenhouse gas (GHG) emissions and non-renewable energy use (NREU).[6] Furthermore, PEF exhibits an intrinsically higher gas barrier for oxygen,[7] carbon dioxide[8] and water vapor[9] than PET and is therefore an interesting alternative for packaging applications such as bottles, films and food trays.
References
- ↑ 1.0 1.1 "PEF". Avalon Industries. http://www.avalon-industries.com/web/pages/en/solutions/applications/bio-based-plastics.php.
- ↑ E. de Jong et al., Furandicarboxylic Acid (FDCA), A Versatile Building Block for a Very Interesting Class of Polyesters, in Biobased Monomers, Polymers, and Materials; Eds. Smith, P., et al., 2012
- ↑ J.-G. Rosenboom et al., Bottle-grade polyethylene furanoate from ring-opening polymerisation of cyclic oligomers, Nature Communications, 2018
- ↑ US 2551731 A, Polyesters from heterocyclic components, 1951
- ↑ Top Value Added Chemicals from Biomass
- ↑ A.J.J.E. Eerhart et al., Replacing fossil based PET with biobased PEF; process analysis, energy and GHG balance, Energy Environ. Sci., 2012
- ↑ S.K. Burgess et al., Oxygen sorption and transport in amorphous poly (ethylene furanoate), Polymer, 2014
- ↑ S.K. Burgess et al., Carbon Dioxide Sorption and Transport in Amorphous Poly (ethylene furanoate), Macromolecules, 2015
- ↑ S.K. Burgess et al., Water sorption in poly (ethylene furanoate) compared to poly (ethylene terephthalate). Part 2: Kinetic sorption, Polymer, 2014

