Chemistry:Sapanisertib
From HandWiki
Short description: Chemical compound
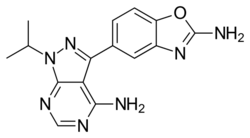 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H15N7O |
| Molar mass | 309.333 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Sapanisertib (also known as MLN0128, INK128 and TAK-228) is an experimental small molecule inhibitor of mTOR which is administered orally. It targets both mTORC1 and mTORC2.[1]
Developed by Millennium Pharmaceuticals,[2][3][4] and is in phase II clinical trials for breast cancer, endometrial cancer, glioblastoma, renal cell carcinoma, and thyroid cancer.[5] The drug has been well tolerated by patients with advanced solid tumours in Phase I trials.[6]
References
- ↑ "In silico Analysis and Molecular Docking Studies of Novel 4-Amino-3- (Isoquinolin-4-yl)-1H-Pyrazolo[3,4-d]Pyrimidine Derivatives as Dual PI3-K/mTOR Inhibitors". Current Drug Discovery Technologies 16 (3): 297–306. 2019. doi:10.2174/1568009618666181102144934. PMID 30387396.
- ↑ "Effects of Neddylation and mTOR Inhibition in Acute Myelogenous Leukemia". Translational Oncology 12 (4): 602–613. April 2019. doi:10.1016/j.tranon.2019.01.001. PMID 30699367.
- ↑ "Impact of Minor Structural Modifications on Properties of a Series of mTOR Inhibitors". ACS Medicinal Chemistry Letters 10 (11): 1561–1567. November 2019. doi:10.1021/acsmedchemlett.9b00401. PMID 31749911.
- ↑ "Synergistic activity of mTORC1/2 kinase and MEK inhibitors suppresses pediatric low-grade glioma tumorigenicity and vascularity". Neuro-Oncology 22 (4): 563–574. April 2020. doi:10.1093/neuonc/noz230. PMID 31841591.
- ↑ "Sapanisertib - Takeda Oncology". Adis Insight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800030541.
- ↑ "Phase I study of the investigational oral mTORC1/2 inhibitor sapanisertib (TAK-228): tolerability and food effects of a milled formulation in patients with advanced solid tumours". ESMO Open 3 (2): e000291. February 2018. doi:10.1136/esmoopen-2017-000291. PMID 29464110.
 |

