Chemistry:Asperlicin
From HandWiki
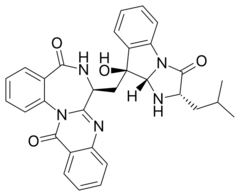
| |
| Names | |
|---|---|
| Preferred IUPAC name
(7S)-7-{[(2S,9S,9aS)-9-Hydroxy-2-(2-methylpropyl)-3-oxo-2,3,9,9a-tetrahydro-1H-imidazo[1,2-a]indol-9-yl]methyl}-6,7-dihydroquinazolino[3,2-a][1,4]benzodiazepine-5,13-dione | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C31H29N5O4 | |
| Molar mass | 535.593 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Asperlicin is a mycotoxin, derived from the fungus Aspergillus alliaceus. It acts as a selective antagonist for the cholecystokinin receptor CCKA,[1][2][3] and has been used as a lead compound for the development of a number of novel CCKA antagonists with potential clinical applications.[4][5][6][7] He et al. 1998 present a synthesis from aryl iodide and vinyl iodide.[8]
References
- ↑ Chang RS, Lotti VJ, Monaghan RL, Birnbaum J, Stapley EO, Goetz MA, Albers-Schönberg G, Patchett AA, Liesch JM, Hensens OD, et al. A potent nonpeptide cholecystokinin antagonist selective for peripheral tissues isolated from Aspergillus alliaceus. Science. 1985 Oct 11;230(4722):177-9. PMID 2994227
- ↑ Goetz MA, Lopez M, Monaghan RL, Chang RS, Lotti VJ, Chen TB. Asperlicin, a novel non-peptidal cholecystokinin antagonist from Aspergillus alliaceus. Fermentation, isolation and biological properties. Journal of Antibiotics (Tokyo). 1985 Dec;38(12):1633-7. PMID 3005212
- ↑ Liesch JM, Hensens OD, Springer JP, Chang RS, Lotti VJ. Asperlicin, a novel non-peptidal cholecystokinin antagonist from Aspergillus alliaceus. Structure elucidation. Journal of Antibiotics (Tokyo). 1985 Dec;38(12):1638-41. PMID 3841533
- ↑ Bock MG, DiPardo RM, Rittle KE, Evans BE, Freidinger RM, Veber DF, Chang RS, Chen TB, Keegan ME, Lotti VJ. Cholecystokinin antagonists. Synthesis of asperlicin analogues with improved potency and water solubility. Journal of Medicinal Chemistry. 1986 Oct;29(10):1941-5. PMID 3761313
- ↑ Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Gould NP, Lundell GF, Homnick CF, Veber DF, et al. Design of nonpeptidal ligands for a peptide receptor: cholecystokinin antagonists. Journal of Medicinal Chemistry. 1987 Jul;30(7):1229-39. PMID 2885419
- ↑ Van der Bent A, Ter Laak AM, IJzerman AP, Soudijn W. Molecular modelling of asperlicin derived cholecystokinin A receptor antagonists. European Journal of Pharmacology. 1992 Aug 3;226(4):327-34. PMID 1397061
- ↑ Lattmann E, Billington DC, Poyner DR, Howitt SB, Offel M. Synthesis and evaluation of asperlicin analogues as non-peptidal cholecystokinin-antagonists. Drug Design and Discovery. 2001;17(3):219-30. PMID 11469752
- ↑ Nakamura, Itaru; Yamamoto, Yoshinori (2004-02-21). "Transition-Metal-Catalyzed Reactions in Heterocyclic Synthesis". Chemical Reviews (American Chemical Society (ACS)) 104 (5): 2127–2198. doi:10.1021/cr020095i. ISSN 0009-2665. PMID 15137788.
 |

