Chemistry:1,2-Bis(diphenylphosphino)ethane
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Ethane-1,2-diyl)bis(diphenylphosphane) | |
| Other names
1,2-Bis(diphenylphosphino)ethane
Diphos Dppe | |
| Identifiers | |
3D model (JSmol)
|
|
| 761261 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| 9052 | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C26H24P2 | |
| Molar mass | 398.42 g/mol |
| Melting point | 140 to 142 °C (284 to 288 °F; 413 to 415 K) |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H302, H315, H319, H332, H335, H410 | |
| P261, P264, P270, P271, P273, P280, P301+312, P302+352, P304+312, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,2-Bis(diphenylphosphino)ethane (dppe) is an organophosphorus compound with the formula (Ph2PCH2)2 (Ph = phenyl). It is a commonly used bidentate ligand in coordination chemistry. It is a white solid that is soluble in organic solvents.
Preparation
The preparation of dppe is by the alkylation of NaPPh2:[1][2]
- P(C6H5)3 + 2 Na → NaP(C6H5)2 + NaC6H5
NaP(C6H5)2, which is readily air-oxidized, is treated with 1,2-dichloroethane (ClCH2CH2Cl) to give dppe:
- 2 NaP(C6H5)2 + ClCH2CH2Cl → (C6H5)2PCH2CH2P(C6H5)2 + 2 NaCl
Reactions
The reduction of dppe by lithium to give PhHP(CH2)2PHPh has been reported.[3]
- Ph2P(CH2)2PPh2 + 4 Li → PhLiP(CH2)2PLiPh + 2 PhLi
Hydrolysis gives the bis(secondary phosphine):
- PhLiP(CH2)2PLiPh + 2 PhLi + 4H2O → PhHP(CH2)2PHPh + 4 LiOH + 2 C6H6
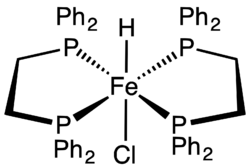 The bis(dppe) complex HFeCl(dppe)2 is one of the most accessible transition metal hydrides.
The bis(dppe) complex HFeCl(dppe)2 is one of the most accessible transition metal hydrides.
Treatment of dppe with conventional oxidants such as hydrogen peroxide (H2O2), aqueous bromine (Br2), etc., produces dppeO in low yield (e.g., 13%) as a result of non-selective oxidation.[4] Selective mono-oxidation of dppe can be achieved by reaction with PhCH2Br to give dppeO.
-
Ph2P(CH2)2PPh2 + PhCH2Br → Ph2P(CH2)2PPh2(CH2Ph)+Br−
()
-
Ph2P(CH2)2PPh2(CH2Ph)+Br− + NaOH + H2O → Ph2P(CH2)2P(O)Ph2
()
Hydrogenation of dppe gives the ligand bis(dicyclohexylphosphino)ethane.
Coordination complexes
Many coordination complexes of dppe are known, and some are used as homogeneous catalysts. Dppe is almost invariably chelating, although there are examples of monodentate (e.g., W(CO)5(dppe)) and of bridging behavior.[5] The natural bite angle is 86°.[6]
Related compounds
References
- ↑ W. Hewertson and H. R. Watson (1962). "283. The preparation of di- and tri-tertiary phosphines". J. Chem. Soc.: 1490–1494. doi:10.1039/JR9620001490.
- ↑ Girolami, G.; Rauchfuss, T.; Angelici, R. Synthesis and Technique in Inorganic Chemistry, 3rd ed.; University Science Books: Sausalito, CA, 1999; pp. 85-92. ISBN:0-935702-48-2
- ↑ Dogan, J.; Schulte, J.B.; Swiegers, G.F.; Wild, S.B. (2000). "Mechanism of Phosphorus-Carbon Bond Cleavage by Lithium in Tertiary Phosphines. An Optimized Synthesis of 1, 2-Bis (phenylphosphino) ethane". J. Org. Chem. 65 (4): 951–957. doi:10.1021/jo9907336. PMID 10814038.
- ↑ Encyclopedia of Reagents for Organic Synthesis 2001 John Wiley & Sons, Ltd
- ↑ Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry: A Comprehensive Text, 4th ed.; Wiley-Interscience Publications: New York, NY, 1980; p.246. ISBN:0-471-02775-8
- ↑ Birkholz (née Gensow), Mandy-Nicole; Freixa, Zoraida; van Leeuwen, Piet W. N. M. (2009). "Bite angle effects of diphosphines in C–C and C–X bond forming cross coupling reactions". Chemical Society Reviews 38 (4): 1099–1118. doi:10.1039/B806211K. PMID 19421583.
 |

