Physics:Sorption
From HandWiki
Revision as of 12:49, 18 April 2022 by imported>MainAI (fixing)
Short description: Physical or chemical process by which one substance becomes attached to another
Sorption is a physical and chemical process by which one substance becomes attached to another. Specific cases of sorption are treated in the following articles:
- Absorption
- "the incorporation of a substance in one state into another of a different state"[1] (e.g., liquids being absorbed by a solid or gases being absorbed by a liquid);
- Adsorption
- The physical adherence or bonding of ions and molecules onto the surface of another phase (e.g., reagents adsorbed to a solid catalyst surface);
- Ion exchange
- An exchange of ions between two electrolytes or between an electrolyte solution and a complex.
The reverse of sorption is desorption.
Sorption rate
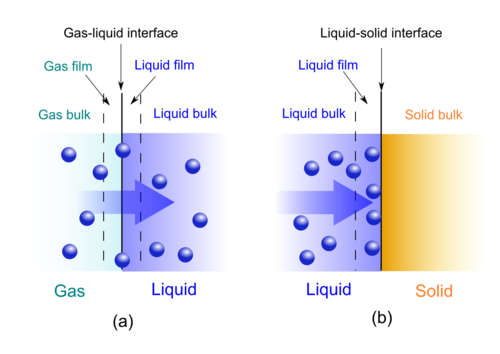
The adsorption and absorption rate of a diluted solute in gas or liquid solution to a surface or interface can be calculated using Fick's laws of diffusion.
See also
- Sorption isotherm
References
- ↑ Crini, Grégorio, ed (2010). Sorption processes and pollution : conventional and non-conventional sorbents for pollutant removal from wastewaters. Besançon: Presses universitaires de Franche-Comté. p. 43. ISBN 978-2848673042.
 |