Biology:KIAA1704
 Generic protein structure example |
KIAA1704, also known as LSR7 (lipopolysaccharide-specific response protein 7), is a protein that in humans is encoded by the GPALPP1 (GPALPP motifs containing 1) gene. The function of KIAA1704 is not yet well understood. KIAA1704 contains one domain of unknown function, DUF3752. The protein contains a conserved, uncharged, repeated motif GPALPP(GF) near the N terminus and an unusual, conserved, mixed charge throughout (alternating readily between positive and negative charges).[1] It is predicted to be localized to the nucleus.[2]
Clinical significance
KIAA1704 has at least a 5 fold expression loss associated with mantle cell lymphoma.[3]
In a second study, researchers used a linkage disequilibrium mapping study of locus 13q13-14 to investigate potential susceptibility for autism over a 1.5 Mb linkage peak, including KIAA1704. A single marker PDTPhase analysis was performed for four SNPs for KIAA1704; however, none of the SNPs were statistically significant in associating the marker with the loci.[4]
An expression study found that KIAA1704 is significantly up-regulated in U937 cells (macrophage-like human cell line) when treated with nicotine.[5]
Properties
| Number of Exons[6] | mRNA sequence length (bp)[6] | Protein sequence length (aa)[6] | Molecular Weight (kD)[2] | Isoelectric point[2] | |
|---|---|---|---|---|---|
| 8 | 1431 | 340 | 38.1 | 5.0526 |
Gene
Location
KIAA1704 is found on the chromosome 13, at locus q14.12, with the genomic sequence starting at 45,563,687 bp and ending at 45,602,405 bp.[6]
Gene Neighborhood
KIAA1704 is located on the positive strand surrounded by 5 nearby genes.
Positive Orientation
- General Transcription Factor IIF (GTF2F2) is further downstream directed in the same orientation. Functionally, it binds to RNA Polymerase II.[7]
Negative Orientation
- Nuclear Fragile X Mental Retardation Protein Interacting Protein 1 (NUFIp1) shows RNA-binding activity with specific nuclear role for FMRP. This is an RNA-binding protein associated with Fragile X. Start sites are antisense to start of KIAA1704.[7]
- Potassium Channel Tetramerisation Domain Containing 4 (KCTD4) proposed to participate in potassium ion transport.[7]
- Tumor protein translationally controlled 1 (TPT1) is involved in calcium binding and microtubule stabilization.[7]
- Small nucleolar RNA, H/ACA box 31 (SNORA31) does not have a known function at this time.[7]
Expression
KIAA1704 has ubiquitous low to moderate expression patterns across body tissues (below 50%)[8]
Promoter
Using GenoMatix ElDorado analysis tools, the promoter was predicted to be 727 base pairs in length projecting into exon 1. There are two predicted transcriptional start sites for this promoter, shown on the adjacent image.[9]
KIAA1704 promoter showed significant histone 3 lysine 4 trimethylation peaks in K562 cells (erythroid cell line). It also showed increased relative expression in erythroid progenitors along with gene neighbors, NUFIP1 and TPT1.[10]
An additional study found that the proximal promoter is one of many thousand direct targets of transcription factor, Myc, in vivo.[11]
mRNA
Splice Variants
According to Ensembl, there are four coding splice variants. None of the alternative splice forms have experimental evidence associated. One splice variant undergoes non-sense mediated decay while another is predicted to splice the gene directly in half and retain amino acids 171–340.[12]
Conservation
NCBI BLAST searches reveal that known mRNA orthologs exist in mammals, reptiles, birds, frogs, and fish with at least 65% sequence identity.[13]
Protein
General Properties
Composition
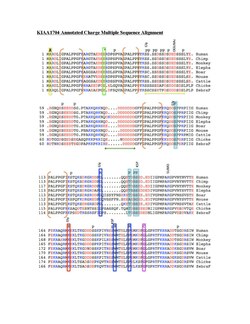
Shown in the table below, KIAA1704 has significantly higher percentages of charged amino acids (D, K, KR, KRED) than the normal human protein and is mostly conserved within its orthologous proteins.[1]
| Compositional Analysis | Amino Acid (AA) Abundance H. sapiens | AA Abundance Mus musculus | AA Abundance Gallus gallus | AA Abundance Xenopus tropicales |
|---|---|---|---|---|
| D++ | 42 (12.4%) | 37 (10.7%) | 35 (10%) | 33 (9.8%) |
| V-- | 6 (1.8%) | 9 (2.6%) | 9 (2.6%) | ----- |
| K+ | 37 (10.9%) | 37 (10.7%) | ----- | ----- |
| L- | 17 (5.0%) | 17 (4.9%) | 19 (5.4%) | ----- |
| KR+ | 62 (18.2%) | 62 (17.9%) | 60 (17.1%) | 56 (16.6%) |
| KRED++ | 134 (39.4%) | 135 (39.0%) | 135 (38.6%) | 122 (36.1%) |
| ED+ | 72 (21.2%) | 73 (21.1%) | 75 (21.4%) | 66 (19.5%) |
| LVIFM- | 54 (15.9%) | 59 (17.1%) | 58 (16.6%) | 57 (16.9%) |
Conservation
KIAA1704 has protein orthologs extending through plants, shown in descending order of identity in the table below. Mammals have the highest level of conservation with 89 percent identity followed by birds, frogs, fish, invertebrates, insects, and plants.[13]
| Genus and Species | Common name | Accession Number | Sequence Length (aa) | Sequence Identity to Human protein (%) | Sequence Similarity to Human Protein (%) | Evolutionary Time to Human Divergence (Million years) |
|---|---|---|---|---|---|---|
| Homo sapiens | Human | NP_061029.2 | 340 | 100 | 100 | 0 |
| Pan troglodytes | Chimpanzee | XP_509661.2 | 340 | 99 | 100 | 6.4 |
| Macaca mulatta | Rhesus monkey | XP_001094145 | 344 | 97 | 98 | 29.2 |
| Loxodonta africana | African savanna elephant | XP_003412655 | 341 | 95 | 98 | 98.8 |
| Sus scrofa | Wild boar | XP_001924228 | 346 | 89 | 95 | 92.4 |
| Mus musculus | Mouse | NP_080453.2 | 346 | 89 | 93 | 94.4 |
| Gallus gallus | Chicken | NP_001006270 | 350 | 67 | 78 | 371.2 |
| Taeniopygia guttata | Zebra finch | XP_002198724 | 342 | 70 | 81 | 400.1 |
| Xenopus tropicales | Western clawed frog | NP_001072786 | 338 | 62 | 74 | 400.1 |
| Xenopus laevis | African clawed frog | NP_001089474 | 337 | 61 | 74 | 661.2 |
| Danio rerio | Zebrafish | NP_001003473 | 405 | 49 | 61 | 782.7 |
| Saccoglossus kowalevskii | Acorn worm | XP_002738946 | 350 | 43 | 60 | 1369 |
| Culex quinquefasciatus | Southern house mosquito | XP_001847636.1 | 335 | 41 | 57 | 782.7 |
| Drosophila ananassae | Fruit fly | XP_001954135 | 348 | 34 | 50 | 1215.8 |
| Glycine max | Soybean | XP_003556198 | 569 | 32 | 51 | 1369 |
| Puccinia graminis | Stem rust | XP_003328471 | 346 | 29 | 46 | 1215.8 |
Conserved domains
Concerning conserved domains, thus far, there does not appear to be much information about conserved motif, GPALPP(GF). This motif represents the neutral segments in this highly charged protein.
DUF3752 is generally found in Eukaryotes and is between 140-163 amino acids in length. It belongs to pfam12572, member of superfamily cl13947[14]
| Conserved Region | H. sapiens Amino Acid Site | Charge (Acidic, Basic, Neutral) |
|---|---|---|
| Poly-serine | 41-49 | Neutral |
| Poly-aspartic Acid | 81-88 | Acidic |
| GPALPP(GF) | 7-14; 32-37; 92-99; 112-119 | Neutral |
| IIGP | 110-113; 146-149 | Neutral |
| DUF3752 | 196-333 | Basic (pI=10.51) |
Information provided by Statistical Analysis of Proteins (SAPS) tool.[1]
Post Translation Modifications
KIAA1704 is predicted by ExPASy tools to undergo several conserved post translational modifications including glycation, o-linked glycosylation, serine phosphorylation, threonine phosphorylation, and several kinase specific phosphorylation (PKC, PKA, and CKII).[2]
Secondary Structure
There are four conserved predicted alpha helices located towards the C terminus of the protein. The N terminus is predicted to be dominated by coiled regions.[15]
Subcellular Localization
ExPASy PSORT predicts 74% chance of being localized to the nucleus.[2]
References
- ↑ 1.0 1.1 1.2 "Methods and algorithms for statistical analysis of protein sequences". Proc. Natl. Acad. Sci. U.S.A. 89 (6): 2002–6. March 1992. doi:10.1073/pnas.89.6.2002. PMID 1549558. Bibcode: 1992PNAS...89.2002B.
- ↑ 2.0 2.1 2.2 2.3 2.4 "ExPASy: The proteomics server for in-depth protein knowledge and analysis". Nucleic Acids Res. 31 (13): 3784–8. July 2003. doi:10.1093/nar/gkg563. PMID 12824418.
- ↑ "Integrated genomic and expression profiling in mantle cell lymphoma: identification of gene-dosage regulated candidate genes". Br. J. Haematol. 143 (2): 210–21. October 2008. doi:10.1111/j.1365-2141.2008.07334.x. PMID 18699851.
- ↑ del Pilar Garavito, Maria (2009). Fine mapping of autism susceptibility loci on chromosome 1q23-24 and chromosome 13q13-q14 (Thesis). Rutgers University. doi:10.7282/T3BK1CJ5.
- ↑ "Microarray analysis of nicotine-induced changes in gene expression in a macrophage-like human cell line". Journal of Periodontal Research 42 (6): 518–26. December 2007. doi:10.1111/j.1600-0765.2007.00976.x. PMID 17956464.
- ↑ 6.0 6.1 6.2 6.3 "Genecards". http://genecards.org/cgi-bin/carddisp.pl?gene=KIAA1704&search=KIAA1704.
- ↑ 7.0 7.1 7.2 7.3 7.4 "NCBI AceView". https://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/index.html.
- ↑ "NCBI GEO: mining tens of millions of expression profiles--database and tools update". Nucleic Acids Res. 35 (Database issue): D760–5. January 2007. doi:10.1093/nar/gkl887. PMID 17099226.
- ↑ "GenoMatix ElDorado". http://www.genomatix.de/online_help/help_eldorado/introduction.html.
- ↑ "MicroRNA-15a and -16-1 act via MYB to elevate fetal hemoglobin expression in human trisomy 13". Proc. Natl. Acad. Sci. U.S.A. 108 (4): 1519–24. January 2011. doi:10.1073/pnas.1018384108. PMID 21205891. Bibcode: 2011PNAS..108.1519S.
- ↑ Kim, Jognhwan (2005). "Genome- wide mapping of DNA- protein interactions in eukaryotes". University of Austin Texas.
- ↑ "Ensembl Genome Browser". http://uswest.ensembl.org/index.html.
- ↑ 13.0 13.1 "Basic local alignment search tool". J. Mol. Biol. 215 (3): 403–10. October 1990. doi:10.1016/S0022-2836(05)80360-2. PMID 2231712.
- ↑ "NCBI Structure". https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?.
- ↑ "SDSC Biology Workbench PELE". http://workbench.sdsc.edu/.