Biology:Myc
| MYC proto-oncogene, bHLH transcription factor | |
|---|---|
| Identifiers | |
| Symbol | MYC |
| Alt. symbols | c-Myc, v-myc |
| NCBI gene | 4609 |
| HGNC | 7553 |
| OMIM | 190080 |
| RefSeq | NM_001354870.1 |
| UniProt | P01106 |
| Other data | |
| Locus | Chr. 8 q24.21 |
| Wikidata | Q20969939 |
| MYCL proto-oncogene, bHLH transcription factor | |
|---|---|
| Identifiers | |
| Symbol | MYCL |
| Alt. symbols | LMYC, MYCL1, bHLHe38, L-Myc, v-myc |
| NCBI gene | 4610 |
| HGNC | 7555 |
| OMIM | 164850 |
| RefSeq | NM_005376 |
| UniProt | P12524 |
| Other data | |
| Locus | Chr. 1 p34.2 |
| Wikidata | Q18029714 |
| MYCN proto-oncogene, bHLH transcription factor | |
|---|---|
| Identifiers | |
| Symbol | MYCN |
| NCBI gene | 4613 |
| HGNC | 7559 |
| OMIM | 164840 |
| RefSeq | NM_005378 |
| UniProt | V |
| Other data | |
| Locus | Chr. 2 p24.3 |
| Wikidata | Q14906753 |
Myc is a family of regulator genes and proto-oncogenes that code for transcription factors. The Myc family consists of three related human genes: c-myc (MYC), l-myc (MYCL), and n-myc (MYCN). c-myc (also sometimes referred to as MYC) was the first gene to be discovered in this family, due to homology with the viral gene v-myc.
In cancer, c-myc is often constitutively (persistently) expressed. This leads to the increased expression of many genes, some of which are involved in cell proliferation, contributing to the formation of cancer.[1] A common human translocation involving c-myc is critical to the development of most cases of Burkitt lymphoma.[2] Constitutive upregulation of Myc genes have also been observed in carcinoma of the cervix, colon, breast, lung and stomach.[1]
Myc is thus viewed as a promising target for anti-cancer drugs.[3] Unfortunately, Myc possesses several features that have rendered it difficult to drug to date, such that any anti-cancer drugs aimed at inhibiting Myc may continue to require perturbing the protein indirectly, such as by targeting the mRNA for the protein rather than via a small molecule that targets the protein itself.[4][5]
c-Myc also plays an important role in stem cell biology and was one of the original Yamanaka factors used to reprogram somatic cells into induced pluripotent stem cells.[6]
In the human genome, C-myc is located on chromosome 8 and is believed to regulate expression of 15% of all genes[7] through binding on enhancer box sequences (E-boxes).
In addition to its role as a classical transcription factor, N-myc may recruit histone acetyltransferases (HATs). This allows it to regulate global chromatin structure via histone acetylation.[8]
Discovery
The Myc family was first established after discovery of homology between an oncogene carried by the Avian virus, Myelocytomatosis (v-myc; P10395) and a human gene over-expressed in various cancers, cellular Myc (c-Myc).[citation needed] Later, discovery of further homologous genes in humans led to the addition of n-Myc and l-Myc to the family of genes.[9]
The most frequently discussed example of c-Myc as a proto-oncogene is its implication in Burkitt's lymphoma. In Burkitt's lymphoma, cancer cells show chromosomal translocations, most commonly between chromosome 8 and chromosome 14 [t(8;14)]. This causes c-Myc to be placed downstream of the highly active immunoglobulin (Ig) promoter region, leading to overexpression of Myc.
Structure
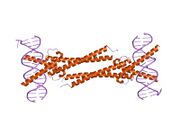
The protein product of Myc family genes all belong to the Myc family of transcription factors, which contain bHLH (basic helix-loop-helix) and LZ (leucine zipper) structural motifs. The bHLH motif allows Myc proteins to bind with DNA, while the leucine zipper TF-binding motif allows dimerization with Max, another bHLH transcription factor.
Myc mRNA contains an IRES (internal ribosome entry site) that allows the RNA to be translated into protein when 5' cap-dependent translation is inhibited, such as during viral infection.
Function
Myc proteins are transcription factors that activate expression of many pro-proliferative genes through binding enhancer box sequences (E-boxes) and recruiting histone acetyltransferases (HATs). Myc is thought to function by upregulating transcript elongation of actively transcribed genes through the recruitment of transcriptional elongation factors.[10] It can also act as a transcriptional repressor. By binding Miz-1 transcription factor and displacing the p300 co-activator, it inhibits expression of Miz-1 target genes. In addition, myc has a direct role in the control of DNA replication.[11] This activity could contribute to DNA amplification in cancer cells.[12]
Myc is activated upon various mitogenic signals such as serum stimulation or by Wnt, Shh and EGF (via the MAPK/ERK pathway).[13] By modifying the expression of its target genes, Myc activation results in numerous biological effects. The first to be discovered was its capability to drive cell proliferation (upregulates cyclins, downregulates p21), but it also plays a very important role in regulating cell growth (upregulates ribosomal RNA and proteins), apoptosis (downregulates Bcl-2), differentiation, and stem cell self-renewal. Nucleotide metabolism genes are upregulated by Myc,[14] which are necessary for Myc induced proliferation[15] or cell growth.[16]
There have been several studies that have clearly indicated Myc's role in cell competition.[17]
A major effect of c-myc is B cell proliferation, and gain of MYC has been associated with B cell malignancies and their increased aggressiveness, including histological transformation.[18] In B cells, Myc acts as a classical oncogene by regulating a number of pro-proliferative and anti-apoptotic pathways, this also includes tuning of BCR signaling and CD40 signaling in regulation of microRNAs (miR-29, miR-150, miR-17-92).[19]
c-Myc induces MTDH(AEG-1) gene expression and in turn itself requires AEG-1 oncogene for its expression.
Myc-nick
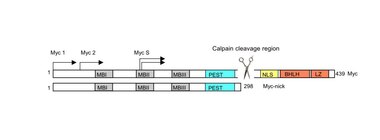
Myc-nick is a cytoplasmic form of Myc produced by a partial proteolytic cleavage of full-length c-Myc and N-Myc.[20] Myc cleavage is mediated by the calpain family of calcium-dependent cytosolic proteases.
The cleavage of Myc by calpains is a constitutive process but is enhanced under conditions that require rapid downregulation of Myc levels, such as during terminal differentiation. Upon cleavage, the C-terminus of Myc (containing the DNA binding domain) is degraded, while Myc-nick, the N-terminal segment 298-residue segment remains in the cytoplasm. Myc-nick contains binding domains for histone acetyltransferases and for ubiquitin ligases.
The functions of Myc-nick are currently under investigation, but this new Myc family member was found to regulate cell morphology, at least in part, by interacting with acetyl transferases to promote the acetylation of α-tubulin. Ectopic expression of Myc-nick accelerates the differentiation of committed myoblasts into muscle cells.
Clinical significance
A large body of evidence shows that Myc genes and proteins are highly relevant for treating tumors.[9] Except for early response genes, Myc universally upregulates gene expression. Furthermore, the upregulation is nonlinear. Genes for which expression is already significantly upregulated in the absence of Myc are strongly boosted in the presence of Myc, whereas genes for which expression is low in the absence Myc get only a small boost when Myc is present.[6]
Inactivation of SUMO-activating enzyme (SAE1 / SAE2) in the presence of Myc hyperactivation results in mitotic catastrophe and cell death in cancer cells. Hence inhibitors of SUMOylation may be a possible treatment for cancer.[21]
Amplification of the MYC gene was found in a significant number of epithelial ovarian cancer cases.[22] In TCGA datasets, the amplification of Myc occurs in several cancer types, including breast, colorectal, pancreatic, gastric, and uterine cancers.[23]
In the experimental transformation process of normal cells into cancer cells, the MYC gene can cooperate with the RAS gene.[24][25]
Expression of Myc is highly dependent on BRD4 function in some cancers.[26][27] BET inhibitors have been used to successfully block Myc function in pre-clinical cancer models and are currently being evaluated in clinical trials.[28]
MYC expression is controlled by a wide variety of noncoding RNAs, including miRNA, lncRNA, and circRNA. Some of these RNAs have been shown to be specific for certain types of human tissues and tumors.[29] Changes in the expression of such RNAs can potentially be used to develop targeted tumor therapy.
Animal models
In Drosophila Myc is encoded by the diminutive locus, (which was known to geneticists prior to 1935).[30] Classical diminutive alleles resulted in a viable animal with small body size. Drosophila has subsequently been used to implicate Myc in cell competition,[31] endoreplication,[32] and cell growth.[33]
During the discovery of Myc gene, it was realized that chromosomes that reciprocally translocate to chromosome 8 contained immunoglobulin genes at the break-point. To study the mechanism of tumorigenesis in Burkitt lymphoma by mimicking expression pattern of Myc in these cancer cells, transgenic mouse models were developed. Myc gene placed under the control of IgM heavy chain enhancer in transgenic mice gives rise to mainly lymphomas. Later on, in order to study effects of Myc in other types of cancer, transgenic mice that overexpress Myc in different tissues (liver, breast) were also made. In all these mouse models overexpression of Myc causes tumorigenesis, illustrating the potency of Myc oncogene. In a study with mice, reduced expression of Myc was shown to induce longevity, with significantly extended median and maximum lifespans in both sexes and a reduced mortality rate across all ages, better health, cancer progression was slower, better metabolism and they had smaller bodies. Also, Less TOR, AKT, S6K and other changes in energy and metabolic pathways (such as AMPK, more oxygen consumption, more body movements, etc.). The study by John M. Sedivy and others used Cre-Loxp -recombinase to knockout one copy of Myc and this resulted in a "Haplo-insufficient" genotype noted as Myc+/-. The phenotypes seen oppose the effects of normal aging and are shared with many other long-lived mouse models such as CR (calorie restriction) ames dwarf, rapamycin, metformin and resveratrol. One study found that Myc and p53 genes were key to the survival of chronic myeloid leukaemia (CML) cells. Targeting Myc and p53 proteins with drugs gave positive results on mice with CML.[34][35]
Relationship to stem cells
Myc genes play a number of normal roles in stem cells including pluripotent stem cells. In neural stem cells, N-Myc promotes a rapidly proliferative stem cell and precursor-like state in the developing brain, while inhibiting differentiation.[36] In hematopoietic stem cells, Myc controls the balance between self-renewal and differentiation.[37]
c-Myc plays a major role in the generation of induced pluripotent stem cells (iPSCs). It is one of the original factors discovered by Yamanaka et al. to encourage cells to return to a 'stem-like' state alongside transcription factors Oct4, Sox2 and Klf4. It has since been shown that it is possible to generate iPSCs without c-Myc.[38]
Interactions
Myc has been shown to interact with:
- ACTL6A[39]
- BRCA1[40][41][42][43]
- Bcl-2[44]
- Cyclin T1[45]
- CHD8[46]
- DNMT3A[47]
- EP400[48]
- GTF2I[49]
- HTATIP[50]
- let-7[51][52][53]
- MAPK1[44][54][55]
- MAPK8[56]
- MAX[57][58][59][60][61][62][63][64][65][66][67][68][69]
- MLH1[61]
- MYCBP2[70]
- MYCBP[71]
- NMI[40]
- NFYB[72]
- NFYC[73]
- P73[74]
- PCAF[75]
- PFDN5[76][77]
- RuvB-like 1[39][48]
- SAP130[75]
- SMAD2[78]
- SMAD3[78]
- SMARCA4[39][57]
- SMARCB1[60]
- SUPT3H[75]
- TIAM1[79]
- TADA2L[75]
- TAF9[75]
- TFAP2A[80]
- TRRAP[39][58][59][75]
- WDR5[81]
- YY1[82] and
- ZBTB17.[83][84]
- C2orf16[85]
See also
References
- ↑ 1.0 1.1 "Myc". NCBI. https://www.ncbi.nlm.nih.gov/gene/17869.
- ↑ "Sequence analysis of the Myc oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered". Proceedings of the National Academy of Sciences of the United States of America 85 (9): 3052–6. May 1988. doi:10.1073/pnas.85.9.3052. PMID 2834731. Bibcode: 1988PNAS...85.3052F.
- ↑ Begley S (2013-01-09). "DNA pioneer James Watson takes aim at cancer establishments". Reuters. https://www.reuters.com/article/us-usa-cancer-watson-idUSBRE90805N20130109.
- ↑ "Therapeutic Inhibition of Myc in Cancer. Structural Bases and Computer-Aided Drug Discovery Approaches". International Journal of Molecular Sciences 20 (1): 120. December 2018. doi:10.3390/ijms20010120. PMID 30597997.
- ↑ "Drugging the 'undruggable' cancer targets". Nature Reviews. Cancer 17 (8): 502–508. August 2017. doi:10.1038/nrc.2017.36. PMID 28643779.
- ↑ 6.0 6.1 "c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells". Cell 151 (1): 68–79. September 2012. doi:10.1016/j.cell.2012.08.033. PMID 23021216.
- ↑ "Pluripotency redux--advances in stem-cell research". The New England Journal of Medicine 357 (15): 1469–72. October 2007. doi:10.1056/NEJMp078126. PMID 17928593.
- ↑ "N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classical transcription factor". Cancer Research 68 (23): 9654–62. December 2008. doi:10.1158/0008-5472.CAN-08-1961. PMID 19047142.
- ↑ 9.0 9.1 "Targeting MYC Proteins for Tumor Therapy". Annual Review of Cancer Biology 4: 61–75. 2020. doi:10.1146/annurev-cancerbio-030518-055826.
- ↑ "MYC and transcription elongation". Cold Spring Harbor Perspectives in Medicine 4 (1): a020990. January 2014. doi:10.1101/cshperspect.a020990. PMID 24384817.
- ↑ "Non-transcriptional control of DNA replication by c-Myc". Nature 448 (7152): 445–51. July 2007. doi:10.1038/nature05953. PMID 17597761. Bibcode: 2007Natur.448..445D.
- ↑ "Stimulation of methotrexate resistance and dihydrofolate reductase gene amplification by c-myc". Oncogene 6 (8): 1453–7. August 1991. PMID 1886715.
- ↑ "Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation". Cell 36 (2): 241–7. 1984. doi:10.1016/0092-8674(84)90217-4. PMID 6692471.
- ↑ "Global regulation of nucleotide biosynthetic genes by c-Myc". PLOS ONE 3 (7): e2722. July 2008. doi:10.1371/journal.pone.0002722. PMID 18628958. Bibcode: 2008PLoSO...3.2722L.
- ↑ "Direct role of nucleotide metabolism in C-MYC-dependent proliferation of melanoma cells". Cell Cycle 7 (15): 2392–400. August 2008. doi:10.4161/cc.6390. PMID 18677108.
- ↑ "The Interplay between Myc and CTP Synthase in Drosophila". PLOS Genetics 12 (2): e1005867. February 2016. doi:10.1371/journal.pgen.1005867. PMID 26889675.
- ↑ "Myc-driven endogenous cell competition in the early mammalian embryo". Nature 500 (7460): 39–44. August 2013. doi:10.1038/nature12389. PMID 23842495. Bibcode: 2013Natur.500...39C.
- ↑ "Analysis of C-MYC function in normal cells via conditional gene-targeted mutation". Immunity 14 (1): 45–55. January 2001. doi:10.1016/S1074-7613(01)00088-7. PMID 11163229.
- ↑ "miRiad roles for the miR-17-92 cluster in development and disease". Cell 133 (2): 217–22. April 2008. doi:10.1016/j.cell.2008.04.001. PMID 18423194.
- ↑ "Myc-nick: a cytoplasmic cleavage product of Myc that promotes alpha-tubulin acetylation and cell differentiation". Cell 142 (3): 480–93. August 2010. doi:10.1016/j.cell.2010.06.037. PMID 20691906.
- ↑ "A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis". Science 335 (6066): 348–53. January 2012. doi:10.1126/science.1212728. PMID 22157079. Bibcode: 2012Sci...335..348K.
- ↑ "Comprehensive genomic profiling of epithelial ovarian cancer by next generation sequencing-based diagnostic assay reveals new routes to targeted therapies". Gynecologic Oncology 130 (3): 554–9. September 2013. doi:10.1016/j.ygyno.2013.06.019. PMID 23791828.
- ↑ "Identification of druggable cancer driver genes amplified across TCGA datasets". PLOS ONE 9 (5): e98293. 2014. doi:10.1371/journal.pone.0098293. PMID 24874471. Bibcode: 2014PLoSO...998293C.
- ↑ "Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes". Nature 304 (5927): 596–602. 1983. doi:10.1038/304596a0. PMID 6308472. Bibcode: 1983Natur.304..596L.
- ↑ "Tumor induction by ras and myc oncogenes in fetal and neonatal brain: modulating effects of developmental stage and retroviral dose". Acta Neuropathologica 86 (5): 456–65. 1993. doi:10.1007/bf00228580. PMID 8310796.
- ↑ "Regulation of MYC expression and differential JQ1 sensitivity in cancer cells". PLOS ONE 9 (1): e87003. 2014. doi:10.1371/journal.pone.0087003. PMID 24466310. Bibcode: 2014PLoSO...987003F.
- ↑ "The mechanisms behind the therapeutic activity of BET bromodomain inhibition". Molecular Cell 54 (5): 728–36. June 2014. doi:10.1016/j.molcel.2014.05.016. PMID 24905006.
- ↑ "BET bromodomain inhibition as a therapeutic strategy to target c-Myc". Cell 146 (6): 904–17. September 2011. doi:10.1016/j.cell.2011.08.017. PMID 21889194.
- ↑ "The Role of Non-Coding RNAs in the Regulation of the Proto-Oncogene MYC in Different Types of Cancer". Biomedicines 9 (8): 921. July 2021. doi:10.3390/biomedicines9080921. PMID 34440124.
- ↑ "Salivary Chromosome Analysis of the White-Facet Region of Drosophila Melanogaster". Genetics 23 (3): 291–9. May 1938. doi:10.1093/genetics/23.3.291. PMID 17246888.
- ↑ "Drosophila myc regulates organ size by inducing cell competition". Cell 117 (1): 107–16. April 2004. doi:10.1016/S0092-8674(04)00214-4. PMID 15066286.
- ↑ "Drosophila dMyc is required for ovary cell growth and endoreplication". Development 131 (4): 775–86. February 2004. doi:10.1242/dev.00932. PMID 14724122.
- ↑ "Drosophila myc regulates cellular growth during development". Cell 98 (6): 779–90. September 1999. doi:10.1016/S0092-8674(00)81512-3. PMID 10499795. PMC 10176494. https://www.zora.uzh.ch/id/eprint/740/1/Johnston_1999.pdf.
- ↑ "Dual targeting of p53 and c-MYC selectively eliminates leukaemic stem cells". Nature 534 (7607): 341–6. June 2016. doi:10.1038/nature18288. PMID 27281222. PMC 4913876. Bibcode: 2016Natur.534..341A. http://man.ac.uk/gIYpq4.
- ↑ "Scientists identify drugs to target 'Achilles heel' of Chronic Myeloid Leukaemia cells". 2016-06-08. https://www.myscience.org.uk/news/2016/cientists_identify_drugs_to_target_achilles_heel_of_chronic_myeloid_leukaemia_cells-2016-glasgow.
- ↑ "N-myc is essential during neurogenesis for the rapid expansion of progenitor cell populations and the inhibition of neuronal differentiation". Genes & Development 16 (20): 2699–712. October 2002. doi:10.1101/gad.1021202. PMID 12381668.
- ↑ "c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation". Genes & Development 18 (22): 2747–63. November 2004. doi:10.1101/gad.313104. PMID 15545632.
- ↑ "A decade of transcription factor-mediated reprogramming to pluripotency". Nature Reviews. Molecular Cell Biology 17 (3): 183–93. March 2016. doi:10.1038/nrm.2016.8. PMID 26883003.
- ↑ 39.0 39.1 39.2 39.3 "BAF53 forms distinct nuclear complexes and functions as a critical c-Myc-interacting nuclear cofactor for oncogenic transformation". Molecular and Cellular Biology 22 (5): 1307–16. March 2002. doi:10.1128/mcb.22.5.1307-1316.2002. PMID 11839798.
- ↑ 40.0 40.1 "A novel tricomplex of BRCA1, Nmi, and c-Myc inhibits c-Myc-induced human telomerase reverse transcriptase gene (hTERT) promoter activity in breast cancer". The Journal of Biological Chemistry 277 (23): 20965–73. June 2002. doi:10.1074/jbc.M112231200. PMID 11916966.
- ↑ "BRCA1 inhibition of telomerase activity in cultured cells". Molecular and Cellular Biology 23 (23): 8668–90. December 2003. doi:10.1128/mcb.23.23.8668-8690.2003. PMID 14612409.
- ↑ "Inhibition of human telomerase reverse transcriptase gene expression by BRCA1 in human ovarian cancer cells". Biochemical and Biophysical Research Communications 303 (1): 130–6. March 2003. doi:10.1016/s0006-291x(03)00318-8. PMID 12646176.
- ↑ "BRCA1 binds c-Myc and inhibits its transcriptional and transforming activity in cells". Oncogene 17 (15): 1939–48. October 1998. doi:10.1038/sj.onc.1202403. PMID 9788437.
- ↑ 44.0 44.1 "Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation". The Journal of Biological Chemistry 279 (38): 40209–19. September 2004. doi:10.1074/jbc.M404056200. PMID 15210690.
- ↑ "c-Myc recruits P-TEFb for transcription, cellular proliferation and apoptosis". Oncogene 22 (36): 5707–11. August 2003. doi:10.1038/sj.onc.1206800. PMID 12944920.
- ↑ "BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors". Journal of Proteomics 118 (12): 95–111. April 2015. doi:10.1016/j.jprot.2014.09.029. PMID 25452129.
- ↑ "Myc represses transcription through recruitment of DNA methyltransferase corepressor". The EMBO Journal 24 (2): 336–46. January 2005. doi:10.1038/sj.emboj.7600509. PMID 15616584.
- ↑ 48.0 48.1 "The p400 complex is an essential E1A transformation target". Cell 106 (3): 297–307. August 2001. doi:10.1016/s0092-8674(01)00450-0. PMID 11509179.
- ↑ "Direct role for Myc in transcription initiation mediated by interactions with TFII-I". Nature 365 (6444): 359–61. September 1993. doi:10.1038/365359a0. PMID 8377829. Bibcode: 1993Natur.365..359R.
- ↑ "MYC recruits the TIP60 histone acetyltransferase complex to chromatin". EMBO Reports 4 (6): 575–80. June 2003. doi:10.1038/sj.embor.embor861. PMID 12776177.
- ↑ "Widespread microRNA repression by Myc contributes to tumorigenesis". Nature Genetics 40 (1): 43–50. January 2008. doi:10.1038/ng.2007.30. PMID 18066065.
- ↑ "Prediction and preliminary validation of oncogene regulation by miRNAs". BMC Molecular Biology 8: 79. 2007. doi:10.1186/1471-2199-8-79. PMID 17877811.
- ↑ "CRD-BP/IMP1 expression characterizes cord blood CD34+ stem cells and affects c-myc and IGF-II expression in MCF-7 cancer cells". The Journal of Biological Chemistry 280 (20): 20086–93. May 2005. doi:10.1074/jbc.M410036200. PMID 15769738.
- ↑ "MAP kinase binds to the NH2-terminal activation domain of c-Myc". FEBS Letters 353 (3): 281–5. October 1994. doi:10.1016/0014-5793(94)01052-8. PMID 7957875.
- ↑ "Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase". Proceedings of the National Academy of Sciences of the United States of America 94 (14): 7337–42. July 1997. doi:10.1073/pnas.94.14.7337. PMID 9207092. Bibcode: 1997PNAS...94.7337T.
- ↑ "Regulation of c-Myc through phosphorylation at Ser-62 and Ser-71 by c-Jun N-terminal kinase". The Journal of Biological Chemistry 274 (46): 32580–7. November 1999. doi:10.1074/jbc.274.46.32580. PMID 10551811.
- ↑ 57.0 57.1 "Large-scale mapping of human protein-protein interactions by mass spectrometry". Molecular Systems Biology 3: 89. 2007. doi:10.1038/msb4100134. PMID 17353931.
- ↑ 58.0 58.1 "The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc". Molecular and Cellular Biology 20 (2): 556–62. January 2000. doi:10.1128/mcb.20.2.556-562.2000. PMID 10611234.
- ↑ 59.0 59.1 "The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins". Cell 94 (3): 363–74. August 1998. doi:10.1016/s0092-8674(00)81479-8. PMID 9708738.
- ↑ 60.0 60.1 "c-MYC interacts with INI1/hSNF5 and requires the SWI/SNF complex for transactivation function". Nature Genetics 22 (1): 102–5. May 1999. doi:10.1038/8811. PMID 10319872.
- ↑ 61.0 61.1 "Interactions of the DNA mismatch repair proteins MLH1 and MSH2 with c-MYC and MAX". Oncogene 22 (6): 819–25. February 2003. doi:10.1038/sj.onc.1206252. PMID 12584560.
- ↑ "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science 251 (4998): 1211–7. March 1991. doi:10.1126/science.2006410. PMID 2006410. Bibcode: 1991Sci...251.1211B.
- ↑ "JLP: A scaffolding protein that tethers JNK/p38MAPK signaling modules and transcription factors". Proceedings of the National Academy of Sciences of the United States of America 99 (22): 14189–94. October 2002. doi:10.1073/pnas.232310199. PMID 12391307. Bibcode: 2002PNAS...9914189L.
- ↑ "Mlx, a novel Max-like BHLHZip protein that interacts with the Max network of transcription factors". The Journal of Biological Chemistry 274 (51): 36344–50. December 1999. doi:10.1074/jbc.274.51.36344. PMID 10593926.
- ↑ "Mmip1: a novel leucine zipper protein that reverses the suppressive effects of Mad family members on c-myc". Oncogene 16 (9): 1149–59. March 1998. doi:10.1038/sj.onc.1201634. PMID 9528857.
- ↑ "Rox, a novel bHLHZip protein expressed in quiescent cells that heterodimerizes with Max, binds a non-canonical E box and acts as a transcriptional repressor". The EMBO Journal 16 (10): 2892–906. May 1997. doi:10.1093/emboj/16.10.2892. PMID 9184233.
- ↑ "X-ray structures of Myc-Max and Mad-Max recognizing DNA. Molecular bases of regulation by proto-oncogenic transcription factors". Cell 112 (2): 193–205. January 2003. doi:10.1016/s0092-8674(02)01284-9. PMID 12553908.
- ↑ "Differential effects of the widely expressed dMax splice variant of Max on E-box vs initiator element-mediated regulation by c-Myc". Oncogene 18 (15): 2489–98. April 1999. doi:10.1038/sj.onc.1202611. PMID 10229200.
- ↑ "Mlx, a new Max-like bHLHZip family member: the center stage of a novel transcription factors regulatory pathway?". Oncogene 19 (29): 3266–77. July 2000. doi:10.1038/sj.onc.1203634. PMID 10918583.
- ↑ "Identification of a large Myc-binding protein that contains RCC1-like repeats". Proceedings of the National Academy of Sciences of the United States of America 95 (16): 9172–7. August 1998. doi:10.1073/pnas.95.16.9172. PMID 9689053. Bibcode: 1998PNAS...95.9172G.
- ↑ "AMY-1, a novel C-MYC binding protein that stimulates transcription activity of C-MYC". Genes to Cells 3 (8): 549–65. August 1998. doi:10.1046/j.1365-2443.1998.00206.x. PMID 9797456.
- ↑ "Mechanism for the transcriptional repression by c-Myc on PDGF beta-receptor". Journal of Cell Science 114 (Pt 8): 1533–44. April 2001. doi:10.1242/jcs.114.8.1533. PMID 11282029.
- ↑ "Cell cycle-dependent switch of up-and down-regulation of human hsp70 gene expression by interaction between c-Myc and CBF/NF-Y". The Journal of Biological Chemistry 274 (34): 24270–9. August 1999. doi:10.1074/jbc.274.34.24270. PMID 10446203.
- ↑ "p73 Interacts with c-Myc to regulate Y-box-binding protein-1 expression". The Journal of Biological Chemistry 277 (35): 31694–702. August 2002. doi:10.1074/jbc.M200266200. PMID 12080043.
- ↑ 75.0 75.1 75.2 75.3 75.4 75.5 "c-Myc transformation domain recruits the human STAGA complex and requires TRRAP and GCN5 acetylase activity for transcription activation". The Journal of Biological Chemistry 278 (22): 20405–12. May 2003. doi:10.1074/jbc.M211795200. PMID 12660246.
- ↑ "MM-1, a novel c-Myc-associating protein that represses transcriptional activity of c-Myc". The Journal of Biological Chemistry 273 (45): 29794–800. November 1998. doi:10.1074/jbc.273.45.29794. PMID 9792694.
- ↑ "MM-1, a c-Myc-binding protein, is a candidate for a tumor suppressor in leukemia/lymphoma and tongue cancer". The Journal of Biological Chemistry 276 (48): 45137–44. November 2001. doi:10.1074/jbc.M106127200. PMID 11567024.
- ↑ 78.0 78.1 "Direct interaction of c-Myc with Smad2 and Smad3 to inhibit TGF-beta-mediated induction of the CDK inhibitor p15(Ink4B)". Molecular Cell 9 (1): 133–43. January 2002. doi:10.1016/s1097-2765(01)00430-0. PMID 11804592.
- ↑ "Guanine nucleotide exchange factor, Tiam1, directly binds to c-Myc and interferes with c-Myc-mediated apoptosis in rat-1 fibroblasts". The Journal of Biological Chemistry 278 (7): 5132–40. February 2003. doi:10.1074/jbc.M206733200. PMID 12446731.
- ↑ "Transcriptional activation by Myc is under negative control by the transcription factor AP-2". The EMBO Journal 14 (7): 1508–19. April 1995. doi:10.1002/j.1460-2075.1995.tb07137.x. PMID 7729426.
- ↑ "Interaction with WDR5 promotes target gene recognition and tumorigenesis by MYC". Molecular Cell 58 (3): 440–52. May 2015. doi:10.1016/j.molcel.2015.02.028. PMID 25818646.
- ↑ "Inhibition of transcriptional regulator Yin-Yang-1 by association with c-Myc". Science 262 (5141): 1889–92. December 1993. doi:10.1126/science.8266081. PMID 8266081. Bibcode: 1993Sci...262.1889S.
- ↑ "Repression of p15INK4b expression by Myc through association with Miz-1". Nature Cell Biology 3 (4): 392–9. April 2001. doi:10.1038/35070076. PMID 11283613.
- ↑ "An alternative pathway for gene regulation by Myc". The EMBO Journal 16 (18): 5672–86. September 1997. doi:10.1093/emboj/16.18.5672. PMID 9312026.
- ↑ "PSICQUIC View". http://www.ebi.ac.uk/Tools/webservices/psicquic/view/results.xhtml?conversationContext=1.
Further reading
- "EBV Regulates c-MYC, Apoptosis, and Tumorigenicity in Burkitt's Lymphoma". Epstein-Barr Virus and Human Cancer. Current Topics in Microbiology and Immunology. 258. 2001. pp. 153–60. doi:10.1007/978-3-642-56515-1_10. ISBN 978-3-642-62568-8.
- "Function and regulation of the transcription factors of the Myc/Max/Mad network". Gene 277 (1–2): 1–14. October 2001. doi:10.1016/S0378-1119(01)00697-7. PMID 11602341.
- "The proto-oncogene c-myc in hematopoietic development and leukemogenesis". Oncogene 21 (21): 3414–21. May 2002. doi:10.1038/sj.onc.1205400. PMID 12032779.
- "c-MYC: more than just a matter of life and death". Nature Reviews. Cancer 2 (10): 764–76. October 2002. doi:10.1038/nrc904. PMID 12360279.
- "Myc pathways provoking cell suicide and cancer". Oncogene 22 (56): 9007–21. December 2003. doi:10.1038/sj.onc.1207261. PMID 14663479.
- "The great MYC escape in tumorigenesis". Cancer Cell 8 (3): 177–8. September 2005. doi:10.1016/j.ccr.2005.08.005. PMID 16169462.
- "Could MYC induction of mitochondrial biogenesis be linked to ROS production and genomic instability?". Cell Cycle 4 (11): 1465–6. November 2005. doi:10.4161/cc.4.11.2121. PMID 16205115.
- ""Myc'ed messages": myc induces transcription of E2F1 while inhibiting its translation via a microRNA polycistron". PLOS Genetics 3 (8): e146. August 2007. doi:10.1371/journal.pgen.0030146. PMID 17784791.
- "Human immunodeficiency virus activates c-myc and Epstein-Barr virus in human B lymphocytes". Annals of the New York Academy of Sciences 651 (1): 422–32. May 1992. doi:10.1111/j.1749-6632.1992.tb24642.x. PMID 1318011. Bibcode: 1992NYASA.651..422A.
- "Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant". Genes & Development 6 (4): 642–54. April 1992. doi:10.1101/gad.6.4.642. PMID 1559612.
- "DNA-activated protein kinase in Raji Burkitt's lymphoma cells. Phosphorylation of c-Myc oncoprotein". European Journal of Biochemistry 206 (2): 595–603. June 1992. doi:10.1111/j.1432-1033.1992.tb16964.x. PMID 1597196.
- "A phosphorylation site located in the NH2-terminal domain of c-Myc increases transactivation of gene expression". The Journal of Biological Chemistry 266 (35): 23521–4. December 1991. doi:10.1016/S0021-9258(18)54312-X. PMID 1748630.
- "Mapping of the MYC gene to band 8q24.12----q24.13 by R-banding and distal to fra(8)(q24.11), FRA8E, by fluorescence in situ hybridization". Cytogenetics and Cell Genetics 57 (2–3): 109–11. 1991. doi:10.1159/000133124. PMID 1914517.
- "Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc". Science 251 (4998): 1211–7. March 1991. doi:10.1126/science.2006410. PMID 2006410. Bibcode: 1991Sci...251.1211B.
- "Immunochemical detection of proteins related to the human c-myc exon 1". The EMBO Journal 5 (9): 2241–50. September 1986. doi:10.1002/j.1460-2075.1986.tb04491.x. PMID 2430795.
- "Myc oncoproteins are phosphorylated by casein kinase II". The EMBO Journal 8 (4): 1111–9. April 1989. doi:10.1002/j.1460-2075.1989.tb03481.x. PMID 2663470.
- "Sequence analysis of the MYC oncogene involved in the t(8;14)(q24;q11) chromosome translocation in a human leukemia T-cell line indicates that putative regulatory regions are not altered". Proceedings of the National Academy of Sciences of the United States of America 85 (9): 3052–6. May 1988. doi:10.1073/pnas.85.9.3052. PMID 2834731. Bibcode: 1988PNAS...85.3052F.
- "MYC oncogene involved in a t(8;22) chromosome translocation is not altered in its putative regulatory regions". Proceedings of the National Academy of Sciences of the United States of America 84 (9): 2824–8. May 1987. doi:10.1073/pnas.84.9.2824. PMID 3033665. Bibcode: 1987PNAS...84.2824S.
- "Nucleotide sequence 3' to the human c-myc oncogene; presence of a long inverted repeat". Gene 72 (1–2): 105–8. December 1988. doi:10.1016/0378-1119(88)90131-X. PMID 3243428.
- "A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas". Cell 52 (2): 185–95. January 1988. doi:10.1016/0092-8674(88)90507-7. PMID 3277717.
External links
- InterPro signatures for protein family: IPR002418, IPR011598, IPR003327
- The Myc Protein
- NCBI Human Myc protein
- Myc cancer gene
- myc+Proto-Oncogene+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
- Generating iPS Cells from MEFS through Forced Expression of Sox-2, Oct-4, c-Myc, and Klf4
- Drosophila Myc - The Interactive Fly
- FactorBook C-Myc
- PDBe-KB provides an overview of all the structure information available in the PDB for Human Myc proto-oncogene protein
 |