Biology:Drosophila
| Drosophila | |
|---|---|

| |
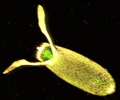
| Drosophila pseudoobscura | |
| Scientific classification | |
| Domain: | Eukaryota |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | Insecta |
| Order: | Diptera |
| Family: | Drosophilidae |
| Subfamily: | Drosophilinae |
| Genus: | Drosophila Fallén, 1823 |
| Type species | |
| Musca funebris Fabricius, 1787
| |
| Subgenera | |
| Synonyms | |
|
Oinopota Kirby & Spence, 1815 | |
Drosophila (/drəˈsɒfɪlə, drɒ-, droʊ-/[1][2]) is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently)[citation needed] pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit. They should not be confused with the Tephritidae, a related family, which are also called fruit flies (sometimes referred to as "true fruit flies"); tephritids feed primarily on unripe or ripe fruit, with many species being regarded as destructive agricultural pests, especially the Mediterranean fruit fly.
One species of Drosophila in particular, D. melanogaster, has been heavily used in research in genetics and is a common model organism in developmental biology. The terms "fruit fly" and "Drosophila" are often used synonymously with D. melanogaster in modern biological literature. The entire genus, however, contains more than 1,500 species[3] and is very diverse in appearance, behavior, and breeding habitat.
Etymology
The term "Drosophila", meaning "dew-loving", is a modern scientific Latin adaptation from Greek words δρόσος, drósos, "dew", and φιλία, philía, "lover".
Morphology
Drosophila species are small flies, typically pale yellow to reddish brown to black, with red eyes. When the eyes (essentially a film of lenses) are removed, the brain is revealed. Drosophila brain structure and function develop and age significantly from larval to adult stage. Developing brain structures make these flies a prime candidate for neuro-genetic research.[4] Many species, including the noted Hawaiian picture-wings, have distinct black patterns on the wings. The plumose (feathery) arista, bristling of the head and thorax, and wing venation are characters used to diagnose the family. Most are small, about 2–4 millimetres (0.079–0.157 in) long, but some, especially many of the Hawaiian species, are larger than a house fly.
Evolution
Detoxification mechanisms
Environmental challenge by natural toxins helped to prepare Drosophilae to detox DDT,[5]:Abstract[5]:1365[5]:1369 by shaping the glutathione S-transferase mechanism[5]:1365[5]:1369 that metabolizes both.[5]:Abstract[6]
Selection
The Drosophila genome is subject to a high degree of selection, especially unusually widespread negative selection compared to other taxa. A majority of the genome is under selection of some sort, and a supermajority of this is occurring in non-coding DNA.[7]
Effective population size has been credibly suggested to positively correlate with the effect size of both negative and positive selection. Recombination is likely to be a significant source of diversity. There is evidence that crossover is positively correlated with polymorphism in D. populations.[7]
Biology
Habitat
Drosophila species are found all around the world, with more species in the tropical regions. Drosophila made their way to the Hawaiian Islands and radiated into over 800 species.[8] They can be found in deserts, tropical rainforest, cities, swamps, and alpine zones. Some northern species hibernate. The northern species D. montana is the best cold-adapted,[9] and is primarily found at high altitudes.[10] Most species breed in various kinds of decaying plant and fungal material, including fruit, bark, slime fluxes, flowers, and mushrooms. Drosophila species that are fruit-breeding are attracted to various products of fermentation, especially ethanol and methanol. Fruits exploited by Drosophila species include those with a high pectin concentration, which is an indicator of how much alcohol will be produced during fermentation. Citrus, morinda, apples, pears, plums, and apricots belong into this category.[11]
The larvae of at least one species, D. suzukii, can also feed in fresh fruit and can sometimes be a pest.[12] A few species have switched to being parasites or predators. Many species can be attracted to baits of fermented bananas or mushrooms, but others are not attracted to any kind of baits. Males may congregate at patches of suitable breeding substrate to compete for the females, or form leks, conducting courtship in an area separate from breeding sites.[citation needed]
Several Drosophila species, including Drosophila melanogaster, D. immigrans, and D. simulans, are closely associated with humans, and are often referred to as domestic species. These and other species (D. subobscura, and from a related genus Zaprionus indianus[13][14][15]) have been accidentally introduced around the world by human activities such as fruit transports.
Reproduction
Males of this genus are known to have the longest sperm cells of any studied organism on Earth, including one species, Drosophila bifurca, that has sperm cells that are 58 mm (2.3 in) long.[16] The cells mostly consist of a long, thread-like tail, and are delivered to the females in tangled coils. The other members of the genus Drosophila also make relatively few giant sperm cells, with that of D. bifurca being the longest.[17] D. melanogaster sperm cells are a more modest 1.8 mm long, although this is still about 35 times longer than a human sperm. Several species in the D. melanogaster species group are known to mate by traumatic insemination.[18]
Drosophila species vary widely in their reproductive capacity. Those such as D. melanogaster that breed in large, relatively rare resources have ovaries that mature 10–20 eggs at a time, so that they can be laid together on one site. Others that breed in more-abundant but less nutritious substrates, such as leaves, may only lay one egg per day. The eggs have one or more respiratory filaments near the anterior end; the tips of these extend above the surface and allow oxygen to reach the embryo. Larvae feed not on the vegetable matter itself, but on the yeasts and microorganisms present on the decaying breeding substrate. Development time varies widely between species (between 7 and more than 60 days) and depends on the environmental factors such as temperature, breeding substrate, and crowding.
Fruit flies lay eggs in response to environmental cycles. Eggs laid at a time (e.g., night) during which likelihood of survival is greater than in eggs laid at other times (e.g., day) yield more larvae than eggs that were laid at those times. Ceteris paribus, the habit of laying eggs at this 'advantageous' time would yield more surviving offspring, and more grandchildren, than the habit of laying eggs during other times. This differential reproductive success would cause D. melanogaster to adapt to environmental cycles, because this behavior has a major reproductive advantage.[19]
Their median lifespan is 35–45 days.[20]
Mating systems
Courtship behavior
The following section is based on the following Drosophila species: Drosophila simulans and Drosophila melanogaster.
Courtship behavior of male Drosophila is an attractive behaviour.[21] Females respond via their perception of the behavior portrayed by the male.[22] Male and female Drosophila use a variety of sensory cues to initiate and assess courtship readiness of a potential mate.[21][22][23] The cues include the following behaviours: positioning, pheromone secretion, following females, making tapping sounds with legs, singing, wing spreading, creating wing vibrations, genitalia licking, bending the stomach, attempt to copulate, and the copulatory act itself.[24][21][22][23] The songs of Drosophila melanogaster and Drosophila simulans have been studied extensively. These luring songs are sinusoidal in nature and varies within and between species.[23]
The courtship behavior of Drosophila melanogaster has also been assessed for sex-related genes, which have been implicated in courtship behavior in both the male and female.[21] Recent experiments explore the role of fruitless (fru) and doublesex (dsx), a group of sex-behaviour linked genes.[25][21]
The fruitless (fru) gene in Drosophila helps regulate the network for male courtship behavior; when a mutation to this gene occurs altered same sex sexual behavior in males is observed.[26] Male Drosophila with the fru mutation direct their courtship towards other males as opposed to typical courtship, which would be directed towards females.[27] Loss of the fru mutation leads back to the typical courtship behavior.[27]
Pheromones
A novel class of pheromones was found to be conserved across the subgenus Drosophila in 11 desert dwelling species.[28] These pheromones are triacylglycerides that are secreted exclusively by males from their ejaculatory bulb and transferred to females during mating. The function of the pheromones is to make the females unattractive to subsequent suitors and thus inhibit courtship by other males.
Polyandry
The following section is based on the following Drosophila species: Drosophila serrata, Drosophila pseudoobscura, Drosophila melanogaster, and Drosophila neotestacea. Polyandry is a prominent mating system among Drosophila.[29][30][31][32] Females mating with multiple sex partners has been a beneficial mating strategy for Drosophila.[29][30][31][32] The benefits include both pre and post copulatory mating. Pre-copulatory strategies are the behaviours associated with mate choice and the genetic contributions, such as production of gametes, that are exhibited by both male and female Drosophila regarding mate choice.[29][30] Post copulatory strategies include sperm competition, mating frequency, and sex-ratio meiotic drive.[29][30][31][32]
These lists are not inclusive. Polyandry among the Drosophila pseudoobscura in North America vary in their number of mating partners.[31] There is a connection between the number of time females choose to mate and chromosomal variants of the third chromosome.[31] It is believed that the presence of the inverted polymorphism is why re-mating by females occurs.[31] The stability of these polymorphisms may be related to the sex-ratio meiotic drive.[32]
However, for Drosophila subobscura, the main mating system is monandry, not normally seen in Drosophila.[33]
Sperm competition
The following section is based on the following Drosophila species: Drosophila melanogaster, Drosophila simulans, and Drosophila mauritiana. Sperm competition is a process that polyandrous Drosophila females use to increase the fitness of their offspring.[34][35][36][37][38] The female Drosophila has two sperm storage organs, the spermathecae and seminal receptacle, that allows her to choose the sperm that will be used to inseminate her eggs.[38] However, some species of Drosophila have evolved to only use one or the other.[39] Females have little control when it comes to cryptic female choice.[37][35] Female Drosophila through cryptic choice, one of several post-copulatory mechanisms, which allows for the detection and expelling of sperm that reduces inbreeding possibilities.[36][35] Manier et al. 2013 has categorized the post copulatory sexual selection of Drosophila melanogaster, Drosophila simulans, and Drosophila mauritiana into the following three stages: insemination, sperm storage, and fertilizable sperm.[37] Among the preceding species there are variations at each stage that play a role in the natural selection process.[37] This sperm competition has been found to be a driving force in the establishment of reproductive isolation during speciation.[40][41]
Parthenogenesis and gynogenesis
Parthenogenesis does not occur in D. melanogaster, but in the gyn-f9 mutant, gynogenesis occurs at low frequency. The natural populations of D. mangebeirai are entirely female, making it the only obligate parthenogenetic species of Drosophila. Parthenogenesis is facultative in parthenogenetica and mercatorum.[42][43]
Laboratory-cultured animals
D. melanogaster is a popular experimental animal because it is easily cultured en masse out of the wild, has a short generation time, and mutant animals are readily obtainable. In 1906, Thomas Hunt Morgan began his work on D. melanogaster and reported his first finding of a white eyed mutant in 1910 to the academic community. He was in search of a model organism to study genetic heredity and required a species that could randomly acquire genetic mutation that would visibly manifest as morphological changes in the adult animal. His work on Drosophila earned him the 1933 Nobel Prize in Medicine for identifying chromosomes as the vector of inheritance for genes. This and other Drosophila species are widely used in studies of genetics, embryogenesis, chronobiology, speciation, neurobiology, and other areas.[citation needed]
However, some species of Drosophila are difficult to culture in the laboratory, often because they breed on a single specific host in the wild. For some, it can be done with particular recipes for rearing media, or by introducing chemicals such as sterols that are found in the natural host; for others, it is (so far) impossible. In some cases, the larvae can develop on normal Drosophila lab medium, but the female will not lay eggs; for these it is often simply a matter of putting in a small piece of the natural host to receive the eggs.[44]
The Drosophila Species Stock Center located at Cornell University in Ithaca, New York, maintains cultures of hundreds of species for researchers.[45]
Use in genetic research
Drosophila is considered one of the most valuable genetic model organisms; both adults and embryos are experimental models.[46] Drosophila is a prime candidate for genetic research because the relationship between human and fruit fly genes is very close.[47] Human and fruit fly genes are so similar, that disease-producing genes in humans can be linked to those in flies. The fly has approximately 15,500 genes on its four chromosomes, whereas humans have about 22,000 genes among their 23 chromosomes. Thus the density of genes per chromosome in Drosophila is higher than the human genome.[48] Low and manageable number of chromosomes make Drosophila species easier to study. These flies also carry genetic information and pass down traits throughout generations, much like their human counterparts.[clarification needed] The traits can then be studied through different Drosophila lineages and the findings can be applied to deduce genetic trends in humans. Research conducted on Drosophila help determine the ground rules for transmission of genes in many organisms.[49][4] Drosophila is a useful in vivo tool to analyze Alzheimer's disease.[50] Rhomboid proteases were first detected in Drosophila but then found to be highly conserved across eukaryotes, mitochondria, and bacteria.[51][52] Melanin's ability to protect DNA against ionizing radiation has been most extensively demonstrated in Drosophila, including in the formative study by Hopwood et al 1985.[53]
Microbiome
Like other animals, Drosophila is associated with various bacteria in its gut. The fly gut microbiota or microbiome seems to have a central influence on Drosophila fitness and life history characteristics. The microbiota in the gut of Drosophila represents an active current research field.
Drosophila species also harbour vertically transmitted endosymbionts, such as Wolbachia and Spiroplasma. These endosymbionts can act as reproductive manipulators, such as cytoplasmic incompatibility induced by Wolbachia or male-killing induced by the D. melanogaster Spiroplasma poulsonii (named MSRO). The male-killing factor of the D. melanogaster MSRO strain was discovered in 2018, solving a decades-old mystery of the cause of male-killing. This represents the first bacterial factor that affects eukaryotic cells in a sex-specific fashion, and is the first mechanism identified for male-killing phenotypes.[54] Alternatively, they may protect theirs hosts from infection. Drosophila Wolbachia can reduce viral loads upon infection, and is explored as a mechanism of controlling viral diseases (e.g. Dengue fever) by transferring these Wolbachia to disease-vector mosquitoes.[55] The S. poulsonii strain of Drosophila neotestacea protects its host from parasitic wasps and nematodes using toxins that preferentially attack the parasites instead of the host.[56][57][58]
Since the Drosophila species is one of the most used model organisms, it was vastly used in genetics. However, the effect abiotic factors,[59] such as temperature, has on the microbiome on Drosophila species has recently been of great interest. Certain variations in temperature have an impact on the microbiome. It was observed that higher temperatures (31 °C) lead to an increase of Acetobacter populations in the gut microbiome of Drosophila melanogaster as compared to lower temperatures (13 °C). In low temperatures (13 °C), the flies were more cold resistant and also had the highest concentration of Wolbachia.[60]
The microbiome in the gut can also be transplanted among organisms. It was found that Drosophila melanogaster became more cold-tolerant when the gut microbiota from Drosophila melanogaster that were reared at low temperatures. This depicted that the gut microbiome is correlated to physiological processes.[61]
Moreover, the microbiome plays a role in aggression, immunity, egg-laying preferences, locomotion and metabolism. As for aggression, it plays a role to a certain degree during courtship. It was observed that germ-free flies were not as competitive compared to the wild-type males. Microbiome of the Drosophila species is also known to promote aggression by octopamine OA signalling. The microbiome has been shown to impact these fruit flies' social interactions, specifically aggressive behaviour that is seen during courtship and mating.[62]
Predators
Drosophila species are prey for many generalist predators, such as robber flies. In Hawaii, the introduction of yellowjackets from mainland United States has led to the decline of many of the larger species. The larvae are preyed on by other fly larvae, staphylinid beetles, and ants.[citation needed]
Neurochemistry
As with many Eukaryotes, this genus is known to express SNAREs, and as with several others the components of the SNARE complex are known to be somewhat substitutable: Although the loss of SNAP-25 - a component of neuronal SNAREs - is lethal, SNAP-24 can fully replace it. For another example, an R-SNARE not normally found in synapses can substitute for synaptobrevin.[63]
Immunity
The Spätzle protein is a ligand of Toll.[64][65] In addition to melanin's more commonly known roles in the endoskeleton and in neurochemistry, melanization is one step in the immune responses to some pathogens.[64][65] Dudzic et al 2019 additionally find a large number of shared serine protease messengers between Spätzle/Toll and melanization and a large amount of crosstalk between these pathways.[64][65]
Systematics
|
The genus Drosophila as currently defined is paraphyletic (see below) and contains 1,450 described species,[3][66] while the total number of species is estimated at thousands.[67] The majority of the species are members of two subgenera: Drosophila (about 1,100 species) and Sophophora (including D. (S.) melanogaster; around 330 species).
The Hawaiian species of Drosophila (estimated to be more than 500, with roughly 380 species described) are sometimes recognized as a separate genus or subgenus, Idiomyia,[3][68] but this is not widely accepted. About 250 species are part of the genus Scaptomyza, which arose from the Hawaiian Drosophila and later recolonized continental areas.
Evidence from phylogenetic studies suggests these genera arose from within the genus Drosophila:[69][70]
- Liodrosophila Duda, 1922
- Mycodrosophila Oldenburg, 1914
- Samoaia Malloch, 1934
- Scaptomyza Hardy, 1849
- Zaprionus Coquillett, 1901
- Zygothrica Wiedemann, 1830
- Hirtodrosophila Duda, 1923 (position uncertain)
Several of the subgeneric and generic names are based on anagrams of Drosophila, including Dorsilopha, Lordiphosa, Siphlodora, Phloridosa, and Psilodorha.
Genetics
Drosophila species are extensively used as model organisms in genetics (including population genetics), cell biology, biochemistry, and especially developmental biology. Therefore, extensive efforts are made to sequence drosphilid genomes. The genomes of these species have been fully sequenced:[71]
- Drosophila (Sophophora) melanogaster
- Drosophila (Sophophora) simulans
- Drosophila (Sophophora) sechellia
- Drosophila (Sophophora) yakuba
- Drosophila (Sophophora) erecta
- Drosophila (Sophophora) ananassae
- Drosophila (Sophophora) pseudoobscura
- Drosophila (Sophophora) persimilis
- Drosophila (Sophophora) willistoni
- Drosophila (Drosophila) mojavensis
- Drosophila (Drosophila) virilis
- Drosophila (Drosophila) grimshawi
The data have been used for many purposes, including evolutionary genome comparisons. D. simulans and D. sechellia are sister species, and provide viable offspring when crossed, while D. melanogaster and D. simulans produce infertile hybrid offspring. The Drosophila genome is often compared with the genomes of more distantly related species such as the honeybee Apis mellifera or the mosquito Anopheles gambiae.
The modEncode consortium is currently sequencing eight more Drosophila genomes,[72] and even more genomes are being sequenced by the i5K consortium.[73]
Curated data are available at FlyBase.
The Drosophila 12 Genomes Consortium – led by Andrew G. Clark, Michael Eisen, Douglas Smith, Casey Bergman, Brian Oliver, Therese Ann Markow, Thomas Kaufman, Manolis Kellis, William Gelbart, Venky Iyer, Daniel Pollard, Timothy Sackton, Amanda Larracuente, Nadia Singh, and including Wojciech Makalowski, Mohamed Noor, Temple F. Smith, Craig Venter, Peter Keightley, and Leonid Boguslavsky among its contributors – presents ten new genomes and combines those with previously released genomes for D. melanogaster and D. pseudoobscura to analyse the evolutionary history and common genomic structure of the genus. This includes the discovery of transposable elements and illumination of their evolutionary history.[74] Bartolomé et al 2009 find at least 1⁄3 of the TEs in D. melanogaster, D. simulans and D. yakuba have been acquired by horizontal transfer. They find an average of 0.035 HT TEs⁄TE family⁄million years. Bartolomé also finds HT TEs follow other relatedness metrics, with D. melanogaster⇔D. simulans events being twice as common as either of them ⇔ D. yakuba.[74]
See also
- Drosophila hybrid sterility
- Laboratory experiments of speciation
- List of Drosophila species
- Caenorhabditis 'Drosophilae' species supergroup, a group of species generally found on rotten fruits and transported by Drosophila flies
References
- ↑ English Pronouncing Dictionary. Cambridge: Cambridge University Press. 2003. ISBN 978-3-12-539683-8.
- ↑ "Drosophila". Merriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/Drosophila.
- ↑ 3.0 3.1 3.2 "TaxoDros: the database on taxonomy of Drosophilidae". 1999–2006. http://www.taxodros.uzh.ch/.
- ↑ 4.0 4.1 "Restoring Tip60 HAT/HDAC2 Balance in the Neurodegenerative Brain Relieves Epigenetic Transcriptional Repression and Reinstates Cognition". The Journal of Neuroscience 38 (19): 4569–4583. May 2018. doi:10.1523/JNEUROSCI.2840-17.2018. PMID 29654189.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Molecular evolution of glutathione S-transferases in the genus Drosophila". Genetics (Genetics Society of America (OUP)) 177 (3): 1363–1375. November 2007. doi:10.1534/genetics.107.075838. PMID 18039872.
- ↑ "Biochemical characterization of Drosophila glutathione S-transferases D1 and D21". The Journal of Biological Chemistry 269 (45): 27876–27884. November 1994. doi:10.1016/S0021-9258(18)46868-8. PMID 7961718.
- ↑ 7.0 7.1 "Patterns of Selection in Plant Genomes". Annual Review of Ecology, Evolution, and Systematics (Annual Reviews) 44 (1): 31–49. 2013-11-23. doi:10.1146/annurev-ecolsys-110512-135851. ISSN 1543-592X.
- ↑ "Endangered Species: Featured Species: Relict Leopard Frog" (in en-US). Ecological Services Program. U.S. Fish and Wildlife Service. 8 October 2015. https://www.fws.gov/endangered/about/ep_45_2015.html.
- ↑ "Inter and Intraspecific Genomic Divergence in Drosophila montana Shows Evidence for Cold Adaptation". Genome Biology and Evolution 10 (8): 2086–2101. August 2018. doi:10.1093/gbe/evy147. PMID 30010752.
- ↑ Genetic and Phenotypic Divergence in Drosophila virilis and D. montana. Jyväskylä: University of Jyväskylä. 2007. p. 13. https://rmbl.org/modules/Downloads/Publications/Routtu_phd_2007.pdf.
- ↑ Keesey, Ian W.; Hansson, Bill S. (2022-01-07). "Neuroecology of Alcohol Preference in Drosophila". Annual Review of Entomology 67 (1): 261–279. doi:10.1146/annurev-ento-070721-091828. ISSN 0066-4170. PMID 34995092.
- ↑ "Spotted Wing Drosophila (Cherry Vinegar Fly) Drosophila suzukii". Center for Invasive Species Research. http://cisr.ucr.edu/spotted_wing_drosophila_cherry_vinegar_fly.html.
- ↑ "Is Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) currently colonizing the Neotropical Region". Drosophila Information Service 82: 37–39. 1 January 1999. https://www.scienceopen.com/document?vid=3dbd8296-d04c-41a5-9062-57e688fc25c0.
- ↑ "First records of Zaprionus indianus (Diptera, Drosophilidae), a pest species on commercial fruits, from Panama and the United States of America". Florida Entomologist 89 (3): 402–404. September 2006. doi:10.1653/0015-4040(2006)89[402:FROZID2.0.CO;2]. ISSN 0015-4040.
- ↑ "New records of Zaprionus indianus Gupta, 1970 (Diptera, Drosophilidae) in North America and a key to identify some Zaprionus species deposited in the Drosophila Tucson Stock Center". Drosophila Information Service 90: 34–36. 2007. http://www.ou.edu/journals/dis/DIS90/Research/Castrezana3.pdf.
- ↑ "How long is a giant sperm?". Nature 375 (6527): 109. May 1995. doi:10.1038/375109a0. PMID 7753164. Bibcode: 1995Natur.375Q.109P.
- ↑ "Adaptation to long sperm in Drosophila: correlated development of the sperm roller and sperm packaging". Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution 310 (2): 167–178. March 2008. doi:10.1002/jez.b.21167. PMID 17377954.
- ↑ "Twin intromittent organs of Drosophila for traumatic insemination". Biology Letters 3 (4): 401–404. August 2007. doi:10.1098/rsbl.2007.0192. PMID 17519186.
- ↑ "Circadian regulation of egg-laying behavior in fruit flies Drosophila melanogaster". Journal of Insect Physiology 52 (8): 779–785. August 2006. doi:10.1016/j.jinsphys.2006.05.001. PMID 16781727.
- ↑ "Longer lifespan, altered metabolism, and stress resistance in Drosophila from ablation of cells making insulin-like ligands". Proceedings of the National Academy of Sciences of the United States of America 102 (8): 3105–3110. February 2005. doi:10.1073/pnas.0405775102. PMID 15708981. Bibcode: 2005PNAS..102.3105B.
- ↑ 21.0 21.1 21.2 21.3 21.4 "Turning males on: activation of male courtship behavior in Drosophila melanogaster". PLOS ONE 6 (6): e21144. 2011. doi:10.1371/journal.pone.0021144. PMID 21731661. Bibcode: 2011PLoSO...621144P.
- ↑ 22.0 22.1 22.2 "Courtship processing in Drosophila melanogaster. II. An adaptation to selection for receptivity to wingless males". Animal Behaviour 21 (2): 349–358. May 1973. doi:10.1016/S0003-3472(73)80077-6. PMID 4198506.
- ↑ 23.0 23.1 23.2 "Courtship song components affect male and female Drosophila differently". Animal Behaviour 50 (3): 827–839. 1995. doi:10.1016/0003-3472(95)80142-1.
- ↑ "Measurement of Courtship Behavior in Drosophila melanogaster". CSH Protocols 2007 (10): pdb.prot4847. October 2007. doi:10.1101/pdb.prot4847. PMID 21356948.
- ↑ "Modulation of Drosophila male behavioral choice". Proceedings of the National Academy of Sciences of the United States of America 104 (11): 4706–4711. March 2007. doi:10.1073/pnas.0700328104. PMID 17360588. Bibcode: 2007PNAS..104.4706C.
- ↑ "fruitless splicing specifies male courtship behavior in Drosophila". Cell 121 (5): 785–794. June 2005. doi:10.1016/j.cell.2005.04.027. PMID 15935764.
- ↑ 27.0 27.1 "What does the fruitless gene tell us about nature vs. nurture in the sex life of Drosophila?". Fly 11 (2): 139–147. April 2017. doi:10.1080/19336934.2016.1263778. PMID 27880074.
- ↑ "Sex-specific triacylglycerides are widely conserved in Drosophila and mediate mating behavior". eLife 3: e01751. March 2014. doi:10.7554/eLife.01751. PMID 24618898.
- ↑ 29.0 29.1 29.2 29.3 "Polyandry and paternity skew in natural and experimental populations of Drosophila serrata". Molecular Ecology 17 (6): 1589–1596. March 2008. doi:10.1111/j.1365-294X.2008.03693.x. PMID 18266626.
- ↑ 30.0 30.1 30.2 30.3 "Evolution of male and female choice in polyandrous systems". Proceedings. Biological Sciences 284 (1851): 20162174. March 2017. doi:10.1098/rspb.2016.2174. PMID 28330914.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 "Can patterns of chromosome inversions in Drosophila pseudoobscura predict polyandry across a geographical cline?". Ecology and Evolution 4 (15): 3072–3081. August 2014. doi:10.1002/ece3.1165. PMID 25247064.
- ↑ 32.0 32.1 32.2 32.3 "Association of polyandry and sex-ratio drive prevalence in natural populations of Drosophila neotestacea". Proceedings. Biological Sciences 280 (1769): 20131397. October 2013. doi:10.1098/rspb.2013.1397. PMID 24004936.
- ↑ "What use is an infertile sperm? A comparative study of sperm-heteromorphic Drosophila". Evolution; International Journal of Organic Evolution 62 (2): 374–385. February 2008. doi:10.1111/j.1558-5646.2007.00280.x. PMID 18053077.
- ↑ "Rapid diversification of sperm precedence traits and processes among three sibling Drosophila species". Evolution; International Journal of Organic Evolution 67 (8): 2348–2362. August 2013. doi:10.1111/evo.12117. PMID 23888856.
- ↑ 35.0 35.1 35.2 "Female x male interactions in Drosophila sperm competition". Science 283 (5399): 217–220. January 1999. doi:10.1126/science.283.5399.217. PMID 9880253.
- ↑ 36.0 36.1 "Sperm competitive ability and genetic relatedness in Drosophila melanogaster: similarity breeds contempt". Evolution; International Journal of Organic Evolution 56 (9): 1789–1795. September 2002. doi:10.1111/j.0014-3820.2002.tb00192.x. PMID 12389723.
- ↑ 37.0 37.1 37.2 37.3 "An analytical framework for estimating fertilization bias and the fertilization set from multiple sperm-storage organs". The American Naturalist 182 (4): 552–561. October 2013. doi:10.1086/671782. PMID 24021407. https://www.zora.uzh.ch/id/eprint/113525/1/Manier%20etal%20AmNat%202013.pdf.
- ↑ 38.0 38.1 "Multiple mechanisms of cryptic female choice act on intraspecific male variation in Drosophila simulans". Behavioral Ecology and Sociobiology 70 (4): 519–532. 2016. doi:10.1007/s00265-016-2069-3. http://urn.fi/URN:NBN:fi:jyu-201603211902.
- ↑ "Evolution of Multiple Kinds of Female Sperm-Storage Organs in Drosophila". Evolution; International Journal of Organic Evolution 53 (6): 1804–1822. December 1999. doi:10.2307/2640442. PMID 28565462.
- ↑ "How sexual selection can drive the evolution of costly sperm ornamentation". Nature 533 (7604): 535–538. May 2016. doi:10.1038/nature18005. PMID 27225128. Bibcode: 2016Natur.533..535L. https://zenodo.org/record/1000843.
- ↑ "Costs and benefits of giant sperm and sperm storage organs in Drosophila melanogaster". Journal of Evolutionary Biology 32 (11): 1300–1309. November 2019. doi:10.1111/jeb.13529. PMID 31465604.
- ↑ Markow, Therese Ann (2013-04-01). "Parents Without Partners: Drosophila as a Model for Understanding the Mechanisms and Evolution of Parthenogenesis" (in en). G3: Genes, Genomes, Genetics 3 (4): 757–762. doi:10.1534/g3.112.005421. ISSN 2160-1836. PMID 23550124.
- ↑ Loppin, Benjamin; Dubruille, Raphaëlle; Horard, Béatrice (August 2015). "The intimate genetics of Drosophila fertilization" (in en). Open Biology 5 (8): 150076. doi:10.1098/rsob.150076. ISSN 2046-2441. PMID 26246493.
- ↑ "Drosophila Fallén, 1823" (in en). Global Biodiversity Information Facility (GBIF). https://www.gbif.org/species/112842957.
- ↑ "The National Drosophila Species Stock Center". College of Agriculture and Life Science, Cornell University. http://blogs.cornell.edu/drosophila/.
- ↑ "Global analysis of RNA-binding protein dynamics by comparative and enhanced RNA interactome capture". Nature Protocols (Nature Portfolio) 16 (1): 27–60. January 2021. doi:10.1038/s41596-020-00404-1. PMID 33208978. (JIPP ORCID: 0000-0001-5395-6549). (MN ORCID: 0000-0002-4834-8888). (SM ORCID: 0000-0003-2640-9560).
- ↑ "Why use the fly in research?". yourgenome.org. https://www.yourgenome.org/facts/why-use-the-fly-in-research.
- ↑ "Drosophila as a model organism". Model Organism Encyclopedia of DNA Elements (modENCODE). http://modencode.sciencemag.org/drosophila/introduction. Retrieved 2019-11-19.
- ↑ "Drosophila – a versatile model in biology & medicine". Materials Today 14 (5): 190–195. May 2011. doi:10.1016/S1369-7021(11)70113-4.
- ↑ "Drosophila melanogaster as a model organism for Alzheimer's disease". Molecular Neurodegeneration 8: 35. November 2013. doi:10.1186/1750-1326-8-35. PMID 24267573.
- ↑ "Rhomboid proteases and their biological functions". Annual Review of Genetics (Annual Reviews) 42 (1): 191–210. 2008. doi:10.1146/annurev.genet.42.110807.091628. PMID 18605900.
- ↑ "The rhomboid-like superfamily: molecular mechanisms and biological roles". Annual Review of Cell and Developmental Biology (Annual Reviews) 30 (1): 235–254. 2014-10-11. doi:10.1146/annurev-cellbio-100913-012944. PMID 25062361. https://ora.ox.ac.uk/objects/uuid:eb7bd447-12a5-421d-b643-b22a82491652.
- ↑ "Melanin is Effective Radioprotector against Chronic Irradiation and Low Radiation Doses". IRPA Regional Congress on Radiation Protection in Central Europe: Radiation Protection and Health. Dubrovnik (Croatia): Croatian Radiation Protection Association. May 2001. p. 35 (of 268).
- ↑ "Male-killing toxin in a bacterial symbiont of Drosophila". Nature 557 (7704): 252–255. May 2018. doi:10.1038/s41586-018-0086-2. PMID 29720654. Bibcode: 2018Natur.557..252H.
- Lay summary in: "Mystery solved: The bacterial protein that kills male fruit flies" (in French). École polytechnique fédérale de Lausanne (EPFL). 5 July 2018. https://actu.epfl.ch/news/mystery-solved-the-bacterial-protein-that-kills--3/.
- ↑ "Wolbachia". World Mosquito Program (WMP). Melbourne, Australia: Monash University. http://www.eliminatedengue.com/our-research/wolbachia.
- ↑ "Macroevolutionary persistence of heritable endosymbionts: acquisition, retention and expression of adaptive phenotypes in Spiroplasma". Molecular Ecology 24 (14): 3752–3765. July 2015. doi:10.1111/mec.13261. PMID 26053523.
- ↑ "A ribosome-inactivating protein in a Drosophila defensive symbiont". Proceedings of the National Academy of Sciences of the United States of America 113 (2): 350–355. January 2016. doi:10.1073/pnas.1518648113. PMID 26712000. Bibcode: 2016PNAS..113..350H.
- ↑ "Generality of toxins in defensive symbiosis: Ribosome-inactivating proteins and defense against parasitic wasps in Drosophila". PLOS Pathogens 13 (7): e1006431. July 2017. doi:10.1371/journal.ppat.1006431. PMID 28683136.
- ↑ "Abiotic Factors". Encyclopedia of Entomology. Dordrecht: Kluwer Academic Publishers. 2004. pp. 7. doi:10.1007/0-306-48380-7_8. ISBN 0-7923-8670-1.
- ↑ "Strong responses of Drosophila melanogaster microbiota to developmental temperature". Fly 12 (1): 1–12. January 2018. doi:10.1080/19336934.2017.1394558. PMID 29095113.
- ↑ "Seasonal shifts in the insect gut microbiome are concurrent with changes in cold tolerance and immunity" (in en). Functional Ecology 32 (10): 2357–2368. October 2018. doi:10.1111/1365-2435.13153. ISSN 0269-8463. https://ir.lib.uwo.ca/cgi/viewcontent.cgi?article=1103&context=biologypub.
- ↑ "Wolbachia Influences the Production of Octopamine and Affects Drosophila Male Aggression". Applied and Environmental Microbiology 81 (14): 4573–4580. July 2015. doi:10.1128/AEM.00573-15. PMID 25934616. Bibcode: 2015ApEnM..81.4573R.
- ↑ "SNARE protein structure and function". Annual Review of Cell and Developmental Biology (Annual Reviews) 19 (1): 493–517. 2003. doi:10.1146/annurev.cellbio.19.110701.155609. PMID 14570579.
- ↑ 64.0 64.1 64.2 "Sexual Dimorphisms in Innate Immunity and Responses to Infection in Drosophila melanogaster". Frontiers in Immunology (Frontiers Media) 10: 3075. 2020-01-31. doi:10.3389/fimmu.2019.03075. PMID 32076419.
- ↑ 65.0 65.1 65.2 "Immune properties of invertebrate phenoloxidases". Developmental and Comparative Immunology (Elsevier) 122: 104098. September 2021. doi:10.1016/j.dci.2021.104098. PMID 33857469.
- ↑ Drosophila: A guide to species identification and use. London: Elsevier. 2005. ISBN 978-0-12-473052-6.
- ↑ Evolution. Cornell University Press. 1999. ISBN 978-0-8014-8594-7.[page needed]
- ↑ Drosophilidae (Diptera). World Catalogue of Insects. 2008. ISBN 978-87-88757-88-0.[page needed]
- ↑ "Out of Hawaii: the origin and biogeography of the genus Scaptomyza (Diptera: Drosophilidae)". Biology Letters 4 (2): 195–199. April 2008. doi:10.1098/rsbl.2007.0575. PMID 18296276.
- ↑ "Phylogeny of Drosophilinae (Diptera: Drosophilidae), with comments on combined analysis and character support". Molecular Phylogenetics and Evolution 24 (2): 249–264. August 2002. doi:10.1016/s1055-7903(02)00226-9. PMID 12144760.
- ↑ "12 Drosophila Genomes Project". Lawrence Berkeley National Laboratory. http://rana.lbl.gov/drosophila/index.html.
- ↑ "modEncode Comparative Genomics white paper". Model Organism Encyclopedia of DNA Elements (modENCODE). ENCODE. http://www.genome.gov/Pages/Research/Sequencing/SeqProposals/modENCODE_ComparativeGenomics_WhitePaper.pdf. Retrieved December 13, 2013.
- ↑ "i5k species nomination summary". i5k Insect and other Arthropod Genome Sequencing Initiative. http://www.arthropodgenomes.org/wiki/i5K_nominations.
- ↑ 74.0 74.1 "Transposable Elements and the Evolution of Insects". Annual Review of Entomology (Annual Reviews) 66 (1): 355–372. January 2021. doi:10.1146/annurev-ento-070720-074650. PMID 32931312. https://hal.archives-ouvertes.fr/hal-03376520/file/Gilbert_et_al_30Jan20_text%2BFig.pdf.
External links
- "Fly Base". Indiana University. http://flybase.bio.indiana.edu/. FlyBase is a comprehensive database for information on the genetics and molecular biology of Drosophila. It includes data from the Drosophila Genome Projects and data curated from the literature.
- "FlyMine". Department of Genetics. University of Cambridge, United Kingdom. http://www.flymine.org. is an integrated database of genomic, expression and protein data for Drosophila
- University of California, Santa Cruz
- "D. melanogaster". UCSC Genome browser. University of California, Santa Cruz (UCSC). http://genome.ucsc.edu/cgi-bin/hgTracks?db=dm6.
- "Drosophila Stock Center". University of California, Santa Cruz (UCSC). http://stockcenter.ucsd.edu/. breeds hundreds of species and supplies them to researchers
- Lawrence Berkeley National Laboratory
- "Berkeley Drosophila Genome Project (BDGP)". Berkeley, CA: Lawrence Berkeley National Laboratory (LBNL). http://www.fruitfly.org/.
- "Assembly, Alignment and Annotation of 12 Drosophila species". Berkeley, CA: Lawrence Berkeley National Laboratory (LBNL). http://rana.lbl.gov/drosophila/.
- "Drosophila Melanogaster". The Bug Squad. 27 September 2022. http://www.thebugsquad.com/fruit-flies/drosophila-melanogaster.
- "TaxoDros: The database on Taxonomy of Drosophilidae". University of Zürich. http://taxodros.uzh.ch/.
- "The Drosophila Virtual library". 5 May 2010. http://ceolas.org/fly/index.html. is library of Drosophila on the web
- "Annual Drosophila Research Conference". Genetics Society of America (GSA). https://genetics-gsa.org/drosophila/.
- "Fly (Drosophila) Facility". Centre for Cellular and Molecular Platforms Fly facility (C-CAMP). Bangalore, India. http://ccamp.res.in/fly-facility.html. – In India microinjection service for the generation of transgenic lines, Screening Platforms, Drosophila strain development
Wikidata ☰ Q312154 entry
 |