Chemistry:AMG 319
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
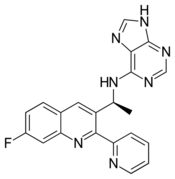
| Formula | C21H16FN7 |
| Molar mass | 385.396 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
AMG 319 is a drug developed by Amgen which acts as an inhibitor of the phosphoinositide 3-kinase enzyme subtype PI3Kδ. It was originally developed as an anti-inflammatory drug with potential applications in the treatment of autoimmune conditions such as rheumatoid arthritis, but subsequent research showed that it inhibits cell proliferation and might potentially have useful anti-cancer effects, and it has been put into clinical trials to assess its safety and tolerability in this application.[1][2]
Mechanism(s) of action
It is a potential immunotherapy because blocking PI3Kδ (PI3K p110δ) eliminates a group of inhibitory immune cells and may allow the immune system to better attack the cancer cells.[3] p110δ inactivation in regulatory T cells unleashes CD8+ cytotoxic T cells.[4]
Clinical trials
Its first clinical trial was a phase I/II study in adults with relapsed or refractory lymphoid malignancies.[5] This was due to run from 2011 to 2013.[citation needed]
In 2015/16 it started a phase II clinical trial as a neoadjuvant therapy for human papillomavirus (HPV) negative head and neck squamous-cell carcinoma (HNSCC) (prior to resection surgery).[3]
See also
References
- ↑ "Discovery and in vivo evaluation of (S)-N-(1-(7-fluoro-2-(pyridin-2-yl)quinolin-3-yl)ethyl)-9H-purin-6-amine (AMG319) and related PI3Kδ inhibitors for inflammation and autoimmune disease". Journal of Medicinal Chemistry 58 (1): 480–511. January 2015. doi:10.1021/jm501624r. PMID 25469863.
- ↑ "First-In-Human Study Of AMG 319, a Highly Selective, Small Molecule Inhibitor Of PI3Kδ, In Adult Patients With Relapsed Or Refractory Lymphoid Malignancies.". 55th ASH Annual Meeting and Exposition. December 2013. https://ash.confex.com/ash/2013/webprogram/Paper57332.html.
- ↑ 3.0 3.1 Clinical trial number NCT02540928 for "AMG 319 in Human PapillomaVirus (HPV) Negative HNSCC" at ClinicalTrials.gov
- ↑ "Inactivation of PI(3)K p110δ breaks regulatory T-cell-mediated immune tolerance to cancer". Nature 510 (7505): 407–411. June 2014. doi:10.1038/nature13444. PMID 24919154. Bibcode: 2014Natur.510..407A.
- ↑ Clinical trial number NCT01300026 for "A Phase 1, First-in-Human Study Evaluating the Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of AMG 319 in Adult Subjects With Relapsed or Refractory Lymphoid Malignancies" at ClinicalTrials.gov
 |

