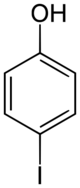
Chemistry:4-Iodophenol
From HandWiki
| |

| |
| Names | |
|---|---|
| IUPAC name
4-Iodophenol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H5IO | |
| Molar mass | 220.009 g·mol−1 |
| Density | 1.8573 g/cm3 (112 °C)[1] |
| Melting point | 93.5 °C (200.3 °F; 366.6 K)[1] |
| Boiling point | 139 °C (282 °F; 412 K)[1] (5 mmHg; decomposes) |
| Acidity (pKa) | 9.33[2] |
| Hazards | |
| GHS pictograms |   [3] [3]
|
| H302, H312, H314 | |
| P280, P305+351+338, P310 | |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
4-Iodophenol (p-iodophenol) is an aromatic organic compound. A colorless solid, it is one of three monoiodophenols. 4-Iodophenol undergoes a variety of coupling reactions in which the iodine substituent is replaced by a new carbon group para to the hydroxy group of the phenol.[3] It is also used to enhance chemiluminescence for detection of cancer cells[4] and in the Eclox assay.
4-Iodophenol can be prepared from 4-aminophenol via the diazonium salt. An alternative synthesis involves reaction of salicylic acid with iodine, followed by decarboxylation.[5]
References
- ↑ 1.0 1.1 1.2 Haynes, p. 3.324
- ↑ Haynes, p. 5.93
- ↑ 3.0 3.1 "4-Iodophenol". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/product/ALDRICH/I10007.
- ↑ "4-Iodophenol". Fisher Scientific. https://www.fishersci.com/shop/products/4-iodophenol-98-thermo-scientific/AAA1345514?searchHijack=true&searchTerm=4-iodophenol-98-thermo-scientific&searchType=Rapid&matchedCatNo=AAA1345514.
- ↑ Dains, F. B.; Eberly, Floyd (1935). "p-Iodophenol". Organic Syntheses 15: 39. doi:10.15227/orgsyn.015.0039.
Cited sources
- Haynes, William M., ed (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.
 |

