Chemistry:Lauryldimethylamine oxide
| |
| Names | |
|---|---|
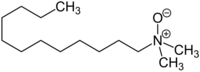
| Preferred IUPAC name
N,N-Dimethyldodecan-1-amine N-oxide | |
| Other names
Lauramine oxide; Dodecyldimethylamine oxide; Dimethyldodecylamine-N-oxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C14H31NO | |
| Molar mass | 229.408 g·mol−1 |
| Appearance | White solid |
| Density | 0.996 g/ml |
| Melting point | 132–133 °C (270–271 °F; 405–406 K) |
| Boiling point | 320 °C (608 °F; 593 K) |
| Hazards | |
| Safety data sheet | [3] |
| GHS pictograms |  [3] [3]
|
| GHS Signal word | Danger[3] |
| H314[3] | |
| P280, P305+351+338, P310[3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lauryldimethylamine oxide (LDAO), also known as dodecyldimethylamine oxide (DDAO), is an amine oxide–based nonionic surfactant, with a C12 (dodecyl) alkyl tail. It is one of the most frequently-used surfactants of this type.[4] Like other amine oxide–based surfactants it is antimicrobial, being effective against common bacteria such as S. aureus and E. coli,[1] however, it is also non-denaturing and may be used to solubilize proteins.
At high concentrations, LDAO forms liquid crystalline phases.[5] Despite having only one polar atom that is able to interact with water – the oxygen atom (the quaternary nitrogen atom is hidden from intermolecular interactions), DDAO is a strongly amphiphilic surfactant: it forms normal micelles and normal liquid crystalline phases. High amphiphilicity of this surfactant can be explained by the fact that it forms not only very strong hydrogen bonds with water: the energy of DDAO – water hydrogen bond is about 50 kJ/mol,[6] but it also has high experimental partition coefficient in non-polar medium, as characterized by experimental logP 5.284[7]
See also
- Myristamine oxide – An analogous compound with a C14 tail
References
- ↑ 1.0 1.1 Birnie, C. R.; Malamud, D.; Schnaare, R. L. (1 September 2000). "Antimicrobial Evaluation of N-Alkyl Betaines and N-Alkyl-N,N-Dimethylamine Oxides with Variations in Chain Length". Antimicrobial Agents and Chemotherapy 44 (9): 2514–2517. doi:10.1128/AAC.44.9.2514-2517.2000. PMID 10952604.
- ↑ Hoffmann, H. (1990). "Correlation between surface and interfacial tensions with micellar structures and properties of surfactant solutions". Interfaces in Condensed Systems. Progress in Colloid & Polymer Science. 83. pp. 16–28. doi:10.1007/BFb0116238. ISBN 978-3-7985-0840-8.
- ↑ 3.0 3.1 3.2 3.3 3.4 Sigma-Aldrich Co., N,N-Dimethyldodecylamine N-oxide. Retrieved on 2017-01-04.
- ↑ Friedli, Floyd E (2001). Detergency of Specialty Surfactants. New York, NY: Dekker. ISBN 978-0-8247-0491-9.
- ↑ Kocherbitov, V.; Söderman, O. (2006). "Hydration of Dimethyldodecylamine-N-Oxide: Enthalpy and Entropy Driven Processes". J. Phys. Chem. B 110 (27): 13649–13655. doi:10.1021/jp060934v. PMID 16821893. http://urn.kb.se/resolve?urn=urn:nbn:se:mau:diva-15244.
- ↑ Kocherbitov, V.; Veryazov, V.; Söderman, O. (2007). "Hydration of Trimethylamine-N-oxide and of Dimethyldodecylamine-N-oxide: An Ab Initio study". J. Molec. Struct. Theochem. 808 (1–3): 111–118. doi:10.1016/j.theochem.2006.12.043.
- ↑ "Lauryldimethylamine oxide | C14H31NO | ChemSpider". http://www.chemspider.com/Chemical-Structure.14688.html.
 |

