Chemistry:Beryllium azide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Beryllium azide
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| Be(N 3) 2 | |
| Molar mass | 93.054 g·mol−1 |
| Appearance | white solid[1] |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
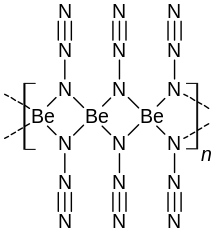
Beryllium azide, Be(N
3)
2, is an inorganic compound. It is the beryllium analog of hydrazoic acid (HN
3).
Synthesis
Beryllium azide has been synthesised by the reaction of beryllium chloride with neat trimethylsilyl azide:[3]
- BeCl
2 + 2 Me
3SiN
3 → Be(N
3)
2 + 2 Me
3SiCl
Alternatively, dimethylberyllium reacts with hydrazoic acid in dry diethyl ether at −116 °C:[1]
- Be(CH
3)
2 + 2 HN
3 → Be(N
3)
2 + 2 CH
4
Structure
Infrared and Raman spectra suggest that beryllium azide consists of infinite chains, with tetrahedrally coordinated beryllium(II) atoms covalently bridged by one end of the azide units.[3]
References
- ↑ 1.0 1.1 Wiberg, E.; Horst, M. (1954). "Beryllium azide, Be(N3)2". Zeitschrift für Naturforschung B 9: 502.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0054.html.
- ↑ 3.0 3.1 Klapötke, T. M.; Schutt, T. (1999). "Synthesis and spectroscopic characterization of beryllium azide and two derivatives". Main Group Metal Chemistry 22 (6): 357–360. doi:10.1515/MGMC.1999.22.6.357.
 |

