Chemistry:Beryllium carbonate
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 1566 |
| |
| |
| Properties | |
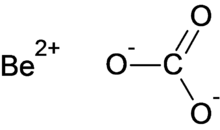
| BeCO 3 | |
| Molar mass | 69.020 g·mol−1 |
| Melting point | 54 °C (129 °F; 327 K) |
| Boiling point | 100 °C (212 °F; 373 K) decomposes |
| 0.36 g/100 mL | |
| Thermochemistry | |
Heat capacity (C)
|
65 J/mol·K[1] |
Std molar
entropy (S |
52 J/mol·K[1] |
Std enthalpy of
formation (ΔfH⦵298) |
-1025 kJ/mol[1] |
Gibbs free energy (ΔfG˚)
|
-948 kJ/mol[1] |
| Hazards | |
| Main hazards | Toxic (T) Irritant (Xi) |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
150 mg/kg (guinea pig) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 0.002 mg/m3 C 0.005 mg/m3 (30 minutes), with a maximum peak of 0.025 mg/m3 (as Be)[2] |
REL (Recommended)
|
Ca C 0.0005 mg/m3 (as Be)[2] |
IDLH (Immediate danger)
|
Ca [4 mg/m3 (as Be)][2] |
| Related compounds | |
Other cations
|
Magnesium carbonate Calcium carbonate Strontium carbonate Barium carbonate Radium carbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Beryllium carbonate is a chemical compound with the chemical formula BeCO
3.
Structures
There are three forms reported, anhydrous, tetrahydrate and basic beryllium carbonate. The anhydrous form is reported to be unstable, decomposing to BeO and carbon dioxide, and requiring storage under CO
2.[4] The tetrahydrate is said to be formed when CO
2 is bubbled through a solution of Be(OH)
2 and is also reported to be similarly unstable.[5]
Preparation
Basic beryllium carbonate is a mixed salt, which can be prepared by the reaction of beryllium sulfate and ammonium carbonate, and contains both carbonate and hydroxide ions, with formula Be
2CO
3(OH)
2.[6] It is believed that in the older literature this is probably what was referred to as beryllium carbonate.[6]
Safety
It may cause irritation. Toxic. It should be handled carefully since several related beryllium compounds are known carcinogens.
Natural occurrence
No formations of purely beryllium carbonate are known to occur naturally. The only Be-rich carbonate mineral currently known is niveolanite.[7]
References
- ↑ 1.0 1.1 1.2 1.3 "Beryllium carbonate". http://chemister.ru/Database/properties-en.php?dbid=1&id=4246.
- ↑ 2.0 2.1 2.2 NIOSH Pocket Guide to Chemical Hazards. "#0054". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0054.html.
- ↑ GHS: GESTIS 082790
- ↑ Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN:0-12-352651-5
- ↑ David Anthony Everest, 1964, The Chemistry of Beryllium, Elsevier Pub. Co.
- ↑ 6.0 6.1 J.E. Macintyre, Dictionary of Inorganic Compounds 1992 CRC Press ISBN:0-412-30120-2
- ↑ "Niveolanite". https://www.mindat.org/min-32289.html.
| H2CO3 | He | ||||||||||||||||
| Li2CO3, LiHCO3 |
BeCO3 | B | C | (NH4)2CO3, NH4HCO3 |
O | F | Ne | ||||||||||
| Na2CO3, NaHCO3, Na3H(CO3)2 |
MgCO3, Mg(HCO3)2 |
Al2(CO3)3 | Si | P | S | Cl | Ar | ||||||||||
| K2CO3, KHCO3 |
CaCO3, Ca(HCO3)2 |
Sc | Ti | V | Cr | MnCO3 | FeCO3 | CoCO3 | NiCO3 | CuCO3 | ZnCO3 | Ga | Ge | As | Se | Br | Kr |
| Rb2CO3 | SrCO3 | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2CO3 | CdCO3 | In | Sn | Sb | Te | I | Xe |
| Cs2CO3, CsHCO3 |
BaCO3 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl2CO3 | PbCO3 | (BiO)2CO3 | Po | At | Rn | |
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La2(CO3)3 | Ce2(CO3)3 | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | Th | Pa | UO2CO3 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |


