Chemistry:Transition metal phosphido complexes
A transition metal phosphido complex is a coordination complex containing a phosphido ligand (R2P, where R = H, organic substituent). With two lone pairs on phosphorus, the phosphido anion (R2P−) is comparable to an amido anion (R2N−), except that the M-P distances are longer and the phosphorus atom is more sterically accessible. For these reasons, phosphido is often a bridging ligand.[1] The -PH2 ion or ligand is also called phosphanide or phosphido ligand.
Synthesis
Phosphido ligands are often installed by salt metathesis reactions. Sources of R2P+ and R2P− are provided by phosphorus halides and alkali metal phosphides respectively. Illustrative of the use of R2PCl-like reagents is the synthesis of a diiron diphosphide:[2]
- Na2Fe2(CO)8 + 2 Ph2PCl → Fe2(PPh2)2(CO)6 + 2 NaCl + 2 CO
The alternative salt metathesis route involves the reaction of alkali metal diorganophosphides with metal halides. A typical phosphide reagent is lithium diphenylphosphide.[3]
Alkali metal phosphides sometimes reduce the metal center.[4]
Another way to generate the transition-metal phosphido complexes are by direct activation of P-H bonds and is mostly seen in the late-transition-metal complexes. For example, the reaction of Vaska's complex analogs with the parent phosphine generate the following transition-metal phosphido complex.[5]

Structure
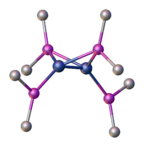
Most complexes of the phosphide ligand can be classified into one of three structural classes:
- those where the phosphide is a terminal ligand and phosphorus is pyramidal,
- those where the phosphide is a terminal ligand and phosphorus is planar,
- those where the phosphide is a bridging ligand and phosphorus is tetrahedral.
Pyramidal terminal phosphido ligands
In most complexes with terminal phosphido ligands, phosphorus is pyramidal, as expected with a stereochemically active lone pair of electrons. The M-P bond length in the pyramidal phosphide complex is longer than the M-P bond length in corresponding transition metal phosphine complexes. The pyramidal phosphido complex. In the complex, the Os-PHPh bond is 0.11 Å longer than the Os-PPh3 and the Os-P-C angle is 113o. The elongated Os-PHPh bond is often attributed to the electronic repulsion of the lone pair and nonbonding electrons on Os.[7] Also, in another ruthenium complex, the Ru-P(Me)Ph bond is 0.17 Å longer than Ru-PH(Me)Ph in the related phosphine ligand version of the complex, [(dmpe)2Ru(H)PH(Me)Ph]+.[8] Additionally electronic repulsion of the P-centered lone pair and metal-based electrons enhance the nucleophilicity of the phosphide ligand. This high basicity and high nucleophilicity leads to the dimerization reaction.
The inversion of configuration at pyramidal terminal phosphides has been observed by 31P NMR spectroscopy.[9][10]
Planar terminal phosphido ligands
Planar terminal phosphido ligands are also known.[11] Terminal planar phosphido ligands engage in M-P multiple bonding. Planar phosphido complexes usually have shorter M-P bonds and wider M-P-R angles. In the tungsten complex, the W-PHPh bond is 0.26 Å shorter than W-PEt3 bond in the same complex, and the W-P-C angle is 140°.[12] In (C
5H
5)
2Hf(PR
2)
2, which can be prepared by salt metathesis from hafnocene dichloride, Hf(IV) is bonded to both planar and pyramidal phosphido ligands. These ligand types interconvert on the NMR timescale corresponding to an activation energy of about 8 kcal/mol. According to X-ray crystallography, the Hf-P distances are 2.488 (planar P) and 2.682 Å (pyramidal P).[13]
Bridging phosphido ligands

In most of its complexes, the phosphido ligand is a bridging ligand. No lone pairs remain on phosphorus. These complexes have the formula [M(μ-PR2)Ln]2. One example is [Fe(μ-PPh2)(CO)3]2.
Potential applications
Metal phosphido complexes are intermediates the catalytic hydrophosphinations, which is a route to organophosphorus compound.
Some late metal hydrophosphination catalysts rely on oxidative addition of a P-H bond. For example, a Pt(0) catalyst that undergoes oxidative addition of a secondary phosphine to form the corresponding Pt(II) phosphido complex, which react with electrophilic alkenes such as acrylonitrile. This P-C bond forming step proceeds through an outer-sphere, Michael-type addition.[15] cAlkene insertion into the metal-hydrogen bond is also invoked in some hydrophosphinations.[16]
Metal phosphides have been used in the synthesis of P-stereogenic phosphines by exploiting the high nucleophilicity in the pyramidal phosphide complex.[17][1]
References
- ↑ 1.0 1.1 Scriban, Corina; Glueck, David S. (March 2006). "Platinum-Catalyzed Asymmetric Alkylation of Secondary Phosphines: Enantioselective Synthesis of P-Stereogenic Phosphines". Journal of the American Chemical Society 128 (9): 2788–2789. doi:10.1021/ja058096q. ISSN 0002-7863. PMID 16506743.
- ↑ Collman, James P.; Rothrock, Richard K.; Finke, Richard G.; Rose-Munch, Francoise (1977). "Metal promoted alkyl migration in a bimetallic complex". Journal of the American Chemical Society 99 (22): 7381–7383. doi:10.1021/ja00464a061.
- ↑ Hey-Hawkins, E. (September 1994). "Bis(cyclopentadienyl)zirconium(IV) or hafnium-(IV) Compounds with Si-, Ge-, Sn-, N-, P-, As-, Sb-, O-, S-, Se-, Te-, or Transition Metal-Centered Anionic Ligands". Chemical Reviews 94 (6): 1661–1717. doi:10.1021/cr00030a009. ISSN 0009-2665.
- ↑ Schäufer, H.; Binder, D. (March 1987). "Übergangsmetallphosphidokomplexe. XI. Diphosphenkomplexe des Typs (R3P)2Ni[η2-(PR′)2] und phosphidoverbrückte Nickel(I)-Komplexe des Typs [R3PNiP(SiMe3)2]2 (Ni–Ni)" (in de). Zeitschrift für anorganische und allgemeine Chemie 546 (3): 55–78. doi:10.1002/zaac.19875460307. ISSN 0044-2313.
- ↑ Ebsworth, E. A. V.; Gould, Robert O.; Mayo, Richard A.; Walkinshaw, Malcolm (1987). "Reactions of phosphine, arsine, and stibine with carbonylbis(triethylphosphine)iridium(I) halides. Part 1. Reactions in toluene; X-ray crystal structures of [Ir(CO)ClH(PEt3) 2 (AsH2) and [Ir(CO)XH(PEt 3 ) 2 (µ-ZH 2 )RuCl 2 (η6-MeC 6H 4CHMe 2-p)](X = Br, Z = P; X = Cl, Z = As)"]. J. Chem. Soc., Dalton Trans. (11): 2831–2838. doi:10.1039/DT9870002831. ISSN 0300-9246. http://xlink.rsc.org/?DOI=DT9870002831.
- ↑ Jones, Richard A.; Lasch, Jon G.; Norman, Nicholas C.; Whittlesey, Bruce R.; Wright, Thomas C. (1983). "Synthesis and X-Ray Crystal Structure of Mo2(μ-t-Bu2P)2(t-Bu2P)2(Mo-Mo); the First Structurally Characterized Binary Transition-Metal Phosphide". Journal of the American Chemical Society 105 (19): 6184–6185. doi:10.1021/ja00357a054.
- ↑ Bohle, D. Scott.; Jones, Tony C.; Rickard, Clifton E. F.; Roper, Warren R. (August 1986). "Terminal phosphido complexes of ruthenium(II) and osmium(II): synthesis, reactivity, and crystal structures of Os(PHPh)Cl(CO)2(PPh3)2 and Os{PH(OMe)Ph}(CO)2(PPh3)2". Organometallics 5 (8): 1612–1619. doi:10.1021/om00139a017. ISSN 0276-7333.
- ↑ Chan, Vincent S.; Stewart, Ian C.; Bergman, Robert G.; Toste, F. Dean (March 2006). "Asymmetric Catalytic Synthesis of P-Stereogenic Phosphines via a Nucleophilic Ruthenium Phosphido Complex". Journal of the American Chemical Society 128 (9): 2786–2787. doi:10.1021/ja058100y. ISSN 0002-7863. PMID 16506742.
- ↑ Baker, R. T.; Krusic, P. J.; Tulip, T. H.; Calabrese, J. C.; Wreford, S. S. (October 1983). "Synthesis and molecular structures of homoleptic dicyclohexylphosphide complexes of the early transition metals". Journal of the American Chemical Society 105 (22): 6763–6765. doi:10.1021/ja00360a061. ISSN 0002-7863.
- ↑ Baker, R. T.; Whitney, J. F.; Wreford, S. S. (August 1983). "Characterization and interconversion of metal-phosphorus single and double bonds: bis(cyclopentadienyl)zirconium and -hafnium bis(diorganophosphide) complexes". Organometallics 2 (8): 1049–1051. doi:10.1021/om50002a022. ISSN 0276-7333.
- ↑ Rosenberg, Lisa (2012). "Metal Complexes of Planar PR2 Ligands: Examining the Carbene Analogy". Coord. Chem. Rev. 256 (5–8): 606-626. doi:10.1016/j.ccr.2011.12.014.
- ↑ Rocklage, Scott M.; Schrock, Richard R.; Churchill, Melvyn Rowen; Wasserman, Harvey J. (October 1982). "Multiple Metal Carbon Bonds. Part 29. Facile Conversion of Tungsten(VI) Neopentylidyne Complexes into Oxo and Imido Neopentylidene Complexes and the Crystal Structure of W(CCMe3)(PHPh)(PEt3)2Cl2". Organometallics 1 (10): 1332–1338. doi:10.1021/om00070a015. ISSN 0276-7333.
- ↑ Baker, R. T.; Whitney, J. F.; Wreford, S. S. (1983). "Characterization and interconversion of metal-phosphorus single and double bonds: Bis(cyclopentadienyl)zirconium and -Hafnium Bis(diorganophosphide) Complexes". Organometallics 2 (8): 1049–1051. doi:10.1021/om50002a022.
- ↑ Ballinas-López, María Gabriela; Padilla-Martínez, Itzia I.; Martínez-Martínez, Francisco J.; Höpfl, Herbert; García-Báez, Efrén V. (2005). "Di-μ-diphenylphosphido-bis[tricarbonyliron(II)] dichloromethane solvate". Acta Crystallographica Section E 61 (8): m1475–m1477. doi:10.1107/S1600536805020982.
- ↑ Scriban, C.; Glueck, D. S.; Zakharov, L. N.; Kassel, W. S.; Dipasquale, A. G.; Golen, J. A.; Rheingold, A. L. (2006). "P−C and C−C Bond Formation by Michael Addition in Platinum-Catalyzed Hydrophosphination and in the Stoichiometric Reactions of Platinum Phosphido Complexes with Activated Alkenes". Organometallics 25 (24): 5757. doi:10.1021/om060631n.
- ↑ Shulyupin, M. O.; Kazankova, M. A.; Beletskaya, I. P. Org. Lett. 2002, 4, 761.Shulyupin, M. O.; Kazankova, M. A.; Beletskaya, I. P. (2002). "Catalytic Hydrophosphination of Styrenes". Organic Letters 4 (5): 761–763. doi:10.1021/ol017238s. PMID 11869121.
- ↑ Chan, Vincent S.; Stewart, Ian C.; Bergman, Robert G.; Toste, F. Dean (March 2006). "Asymmetric Catalytic Synthesis of P -Stereogenic Phosphines via a Nucleophilic Ruthenium Phosphido Complex". Journal of the American Chemical Society 128 (9): 2786–2787. doi:10.1021/ja058100y. ISSN 0002-7863.
 |







