Chemistry:Dysprosium acetylacetonate
| |
| Names | |
|---|---|
| IUPAC name
dysprosium(3+); (''Z'')-4-oxopent-2-en-2-olate
| |
| Other names
Dysprosium(III) 2,4-pentanedionate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C15H21DyO6 | |
| Molar mass | 459.827 g·mol−1 |
| Appearance | Yellow powder |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
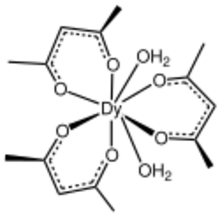
Dysprosium acetylacetonate is a chemical compound of dysprosium with formula Dy(C5H7O2)3(H2O)n.
Preparation and properties
Dysprosium acetylacetonate can be prepared by reacting dysprosium[2] or dysprosium hydride[3] with acetylacetone. Dy(acac)3·EtOH·0.5Hacac (where Hacac represents acetylacetone) can be obtained by electrolysis of dysprosium cathode in ethanol solution of acetylacetone, which can be heated to generate Dy(acac)3 through Dy(acac)3·EtOH.[4] It is a colorless solid. Its anhydrous form is stable in dry atmosphere and it forms a hydrate in humid air.[5] It can form Dy(acac)3·2CH3OH and Dy(acac)3·CH3OH·CH3CN in a methanol solution of acetonitrile.[6]
Applications
Dysprosium acetylacetonate can be used to catalyze the addition reaction of norbornene and carbon tetrachloride.[7] The dihydrate has been characterized by X-ray crystallography.[8]
References
- ↑ Dysprosium acetylacetonate at American Elements
- ↑ J.R. Blackborow, C.R. Eady, E.A.Koerner Von Gustorf, A. Scrivanti, O. Wolfbeis (March 1976). "Chemical syntheses with metal atoms" (in en). Journal of Organometallic Chemistry 108 (3): C32–C34. doi:10.1016/S0022-328X(00)92025-4. https://linkinghub.elsevier.com/retrieve/pii/S0022328X00920254. Retrieved 2021-09-20.
- ↑ Janice M. Koehler, William G. Bos (December 1967). "A novel synthesis of some anhydrous rare earth acetylacetonates" (in en). Inorganic and Nuclear Chemistry Letters 3 (12): 545–548. doi:10.1016/0020-1650(67)80023-0. https://linkinghub.elsevier.com/retrieve/pii/0020165067800230. Retrieved 2021-09-20.
- ↑ Kostyuk, N. N.; Shirokii, V. L.; Vinokurov, I. I.; Maier, N. A. Electrochemical synthesis of dysprosium(III) acetylacetonate complex and its thermolysis. Zhurnal Obshchei Khimii, 1994. 64 (9): 1432-1434. ISSN 0044-460X. (in Russian)
- ↑ Trembovetskii, G. V.; Martynenko, L. I.; Murav'eva, I. A.; Spitsyn, V. Synthesis and study of volatile rare earth acetylacetonates. Doklady Akademii Nauk SSSR, 1984. 277 (6): 1411-1414. ISSN: 0002-3264. (in Russian)
- ↑ Pod'yachev, S. N.; Mustafina, A. R.; Vul'fson, S. G. Structure of tris(acetylacetonato)dysprosium and its monoadduct with acetonitrile in a methanol solution. Zhurnal Neorganicheskoi Khimii, 1994. 39 (1): 67-70. ISSN 0044-457X. (in Russian)
- ↑ R. I. Khusnutdinov, T. M. Oshnyakova (February 2014). "Tetrachloromethane addition to norbornene catalyzed by lanthanide(III) compounds" (in en). Russian Journal of Organic Chemistry 50 (2): 303–305. doi:10.1134/S1070428014020304. ISSN 1070-4280. http://link.springer.com/10.1134/S1070428014020304. Retrieved 2021-09-20.
- ↑ Ilyukhin, A. B.; Gavrikov, A. V.; Dobrokhotova, Zh. V.; Novotortsev, V. M. (2018). "New Solvate Polymorphs of Lanthanide Trisacetylacetonates: Crystal Structures of [Ln(acac)3(H2O)2] · Solv (Ln = Eu, Dy; Solv = THF, H2O + EtOH, MeOH)". Russian Journal of Inorganic Chemistry 63 (9): 1186–1191. doi:10.1134/S003602361809005X.
 |

