Chemistry:Sodium hexanitritocobaltate(III)
| |

| |
| Names | |
|---|---|
| IUPAC name
Sodium hexanitritocobaltate(III)
| |
| Other names
Sodium cobaltinitrite
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
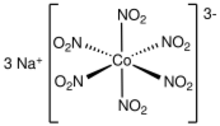
| Na 3[Co(NO 2) 6] | |
| Molar mass | 403.933 g·mol−1 |
| Appearance | Yellow crystals |
| Density | 2.565 g/cm3 |
| Hazards | |
| Safety data sheet | JT Baker MSDS |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H272, H315, H317, H319, H334, H335, H351 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
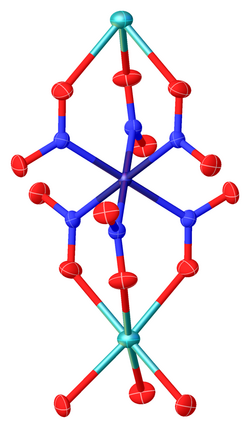
Sodium hexanitritocobaltate(III) is inorganic compound with the formula Na
3[Co(NO
2)
6]. The anion of this yellow-coloured salt consists of the transition metal nitrite complex [Co(NO
2)
6]3−. It was a reagent for the qualitative test for potassium and ammonium ions.[2]
Synthesis and reactions
The compound is prepared by oxidation of cobalt(II) salts in the presence of sodium nitrite:[3]
- 4 [Co(H
2O)
6](NO
3)
2 + O
2 + 24 NaNO
2 → 4 Na
3[Co(NO
2)
6] + 8 NaNO
3 + 4 NaOH + 22 H
2O
Application for analysis of potassium
Although the sodium cobaltinitrite is soluble in water, it forms the basis of a quantitative determination of potassium, thallium, and ammonium ions. Under the recommended reaction conditions the insoluble double salt, K
2Na[Co(NO
2)
6] · H2O is precipitated and weighed.[4] In geochemical analysis, sodium cobaltinitrite is used to distinguish alkali feldspars from plagioclase feldspars in thin section.[5]
See also
References
- ↑ "C&L Inventory". https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/44858.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Glemser, O. (1963). "Sodium Hexanitritocobaltate(III)". in Brauer, G.. Handbook of Preparative Inorganic Chemistry. 1 (2nd ed.). New York, NY: Academic Press. p. 1541.
- ↑ Vogel, A. I. (1951). Quantitative Inorganic Analysis (2nd ed.). Longmans Green and Co..
- ↑ Bailey, E. H.; Stevens, R. E. (1960). "Selective staining of K-feldspar and plagioclase on rock slabs and thin sections". American Mineralogist 45: 1020–1025.
- ↑ Brian N. Figgis; Alexandre N. Soboleva (2001). "Na3Co(NO2)6 at 293 and 10 K". Acta Crystallographica Section C 57 (Pt 8): 885–886. doi:10.1107/S0108270101007995. PMID 11498599.
 |