Chemistry:O-Cresyl glycidyl ether
| |
| Names | |
|---|---|
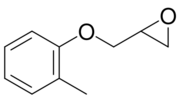
| IUPAC name
2-[(2-Methylphenoxy)methyl]oxirane
| |
| Other names
ortho-Cresyl glycidyl ether; o-CGE; Glycidyl 2-methylphenyl ether
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UN number | 3334 |
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Warning |
| H315, H317, H341, H411 | |
| P201, P202, P261, P264, P272, P273, P280, P281, P302+352, P308+313, P321, P332+313, P333+313, P362, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
o-Cresyl glycidyl ether (ortho-cresyl glycidyl ether, o-CGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether.[2] It has the formula C10H12O2 and the CAS Registry Number 2210-79-9.[3] It is one of a number of glycidyl ethers available commercially that are used to reduce the viscosity of epoxy resins. These are then further used in coatings, sealants, adhesives and elastomers.[4]
Uses
The main use of o-CGE is to reduce the viscosity of epoxy resins.[5][6] These reduced viscosity resins may then be used to formulate coatings including UV cured versions.[7] It is a monofunctional diluent and so in polymer science terms is a chain terminator. Chain extenders (f = 2) and cross linkers (f ≥ 3) are low molecular weight di or tri-functional epoxy diluents. The use of the diluent does effect mechanical properties and microstructure of epoxy resins.[8][9] As it has glycidyl functionality, it is classed as a Reactive diluent.
Other names
o-CGE is known by a number of other names.[10][11] These include:
- Oxirane, 2-[(2-methylphenoxy)methyl]-
- Propane, 1,2-epoxy-3-(o-tolyloxy)-
- Oxirane, [(2-methylphenoxy)methyl]-[12]
- 2-[(2-Methylphenoxy)methyl]oxirane
- 1-(o-Methylphenoxy)-2,3-epoxypropane
Toxicology
The material is a skin irritant and skin sensitizer. The toxicology has been reasonably well studied.[13][14][15][16] It is REACH registered and produced or imported into the European Union in quantities greater than one thousand tonnes per annum.[17]
See also
References
- ↑ "O-Cresyl glycidyl ether" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/16640#section=Safety-and-Hazards.
- ↑ "o-cresyl glycidyl ether" (in en). https://www.wikidata.org/wiki/Q1964115.
- ↑ PubChem. "O-Cresyl glycidyl ether" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/16640.
- ↑ Howarth G.A "Synthesis of a legislation compliant corrosion protection coating system based on urethane, oxazolidine and waterborne epoxy technology" pages 23,24,39 Master of Science Thesis April 1997 Imperial College London
- ↑ Monte, Salvatore J. (1998), Pritchard, Geoffrey, ed., "Diluents and viscosity modifiers for epoxy resins" (in en), Plastics Additives: An A-Z reference, Polymer Science and Technology Series (Dordrecht: Springer Netherlands) 1: pp. 211–216, doi:10.1007/978-94-011-5862-6_24, ISBN 978-94-011-5862-6, https://doi.org/10.1007/978-94-011-5862-6_24, retrieved 2022-03-29
- ↑ Anderson, Lawrence G.; Shawn A. De Saw & Marvis E. Hartman et al., "Flexible coating compositions having improved scratch resistance, coated substrates and methods related thereto", US patent 6987144, published 2006-01-17, assigned to PPG Industries, Ohio Inc.
- ↑ Jung, Ki Sung; Dae Kyu Kim & Jung Wook Kim et al., "UV-curable epoxy resin composition comprising lactone monomer", WO patent application 2003076544, published 2003-09-18, assigned to Luvantix Co. Ltd., application discontinued outside South Korea.
- ↑ Pastarnokienė, Liepa; Jonikaitė-Švėgždienė, Jūratė; Lapinskaitė, Neringa; Kulbokaitė, Rūta; Bočkuvienė, Alma; Kochanė, Tatjana; Makuška, Ričardas (2023-07-01). "The effect of reactive diluents on curing of epoxy resins and properties of the cured epoxy coatings" (in en). Journal of Coatings Technology and Research 20 (4): 1207–1221. doi:10.1007/s11998-022-00737-4. ISSN 1935-3804. https://doi.org/10.1007/s11998-022-00737-4.
- ↑ Khalina, Morteza; Beheshty, Mohammad Hosain; Salimi, Ali (2019-08-01). "The effect of reactive diluent on mechanical properties and microstructure of epoxy resins" (in en). Polymer Bulletin 76 (8): 3905–3927. doi:10.1007/s00289-018-2577-6. ISSN 1436-2449. https://doi.org/10.1007/s00289-018-2577-6.
- ↑ "System of Registries | US EPA" (in en). https://sor.epa.gov/sor_internet/registry/substreg/searchandretrieve/advancedsearch/externalSearch.do?p_type=CASNO&p_value=2210-79-9.
- ↑ "CAS Common Chemistry". https://commonchemistry.cas.org/detail?ref=2210-79-9.
- ↑ "Oxirane, [(2-methylphenoxy)methyl- - MS - Spectrum - SpectraBase"]. https://spectrabase.com/spectrum/7saZDe99lgm.
- ↑ Kanerva, L.; Jolanki, R.; Alanko, K.; Estlander, T. (July 1999). "Patch-test reactions to plastic and glue allergens". Acta Dermato-Venereologica 79 (4): 296–300. doi:10.1080/000155599750010706. ISSN 0001-5555. PMID 10429988.
- ↑ "ICSC 0135 - o-CRESYL GLYCIDYL ETHER". https://inchem.org/documents/icsc/icsc/eics0135.htm.
- ↑ "O-cresyl glycidyl ether | Allergic Contact Dermatitis Database". https://www.contactdermatitisinstitute.com/o-cresyl-glycidyl-ether.php.
- ↑ "O-CRESYL GLYCIDYL ETHER | CAMEO Chemicals | NOAA". https://cameochemicals.noaa.gov/chemical/20057.
- ↑ "Substance Information - ECHA" (in en-GB). https://echa.europa.eu/substance-information/-/substanceinfo/100.016.951.
Further reading
- Epoxy resin technology.. Paul F. Bruins, Polytechnic Institute of Brooklyn. New York: Interscience Publishers. 1968. ISBN 0-470-11390-1. OCLC 182890. https://www.worldcat.org/oclc/182890.
- Flick, Ernest W. (1993). Epoxy resins, curing agents, compounds, and modifiers : an industrial guide. Park Ridge, NJ. ISBN 978-0-8155-1708-5. OCLC 915134542. https://www.worldcat.org/oclc/915134542.
- Lee, Henry (1967). Handbook of epoxy resins. Kris Neville ([2nd, expanded work] ed.). New York: McGraw-Hill. ISBN 0-07-036997-6. OCLC 311631322. https://www.worldcat.org/oclc/311631322.
- "Dow Epoxy Resins". http://nmt.edu/academics/mtls/faculty/mccoy/docs2/chemistry/DowEpoxyResins.pdf.
External Websites
 |

