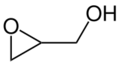
Chemistry:Glycidol
| |
| Names | |
|---|---|
| Preferred IUPAC name
Oxiranylmethanol | |
| Other names
Glycidol
2,3-Epoxy-1-propanol 3-Hydroxypropylene oxide Epoxypropyl alcohol Hydroxymethyl ethylene oxide 2-Hydroxymethyl oxiran | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H6O2 | |
| Molar mass | 74.079 g·mol−1 |
| Appearance | Viscous liquid |
| Density | 1.1143 g/cm3[1] |
| Melting point | −54 °C (−65 °F; 219 K)[3] |
| Boiling point | 167 °C (333 °F; 440 K) (decomposes)[1] |
| miscible[2] | |
| Vapor pressure | 0.9 mmHg (25°C)[2] |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | 66 °C (151 °F; 339 K)[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
420 mg/kg (oral, rat)[3][4] 1980 mg/kg (dermal, rabbit)[3][5] |
LC50 (median concentration)
|
450 ppm (mouse, 4 hr) 580 ppm (rat, 8 hr)[6] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 50 ppm (150 mg/m3)[2] |
REL (Recommended)
|
TWA 25 ppm (75 mg/m3)[2] |
IDLH (Immediate danger)
|
150 ppm[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Glycidol is an organic compound that contains both epoxide and alcohol functional groups. Being bifunctional, it has a variety of industrial uses. The compound is a slightly viscous liquid that is slightly unstable and is not often encountered in pure form.
Synthesis and applications
Glycidol is prepared by the epoxidation of allyl alcohol.[7]
Glycidol is used as a stabilizer for natural oils and vinyl polymers and as a demulsifier. It is used as a chemical intermediate in the synthesis of glycerol, glycidyl ethers, esters and amines. It is used in surface coatings, chemical synthesis, pharmaceuticals, sanitary chemicals and sterilizing milk of magnesia, and as a gelation agent in solid propellants.[8]
- Alkylation of 2-methylquinazolin-4(3H)-one with glycidol affords diproqualone.
- Dyphylline was made by the alkylation of theophylline with glycidol.
- Diproxadol
Safety
Glycidol is an irritant of the skin, eyes, mucous membranes, and upper respiratory tract. Exposure to glycidol may also cause central nervous system depression, followed by central nervous system stimulation.[9] It is listed as an IARC Group 2A Agent, meaning that it is "probably carcinogenic to humans".[10] In regards to occupational exposures, the Occupational Safety and Health Administration has set a permissible exposure limit at 50 ppm over an eight-hour work shift, while the National Institute for Occupational Safety and Health recommends a limit at 25 ppm over an eight-hour work shift.[11]
Refined edible oils have been shown to contain glycidyl fatty acid esters that are thought to be formed primarily during deodorization; hydrolysis of these compounds in the digestive tract releases free glycidol that proved to be carcinogenic in rats.[12]
See also
References
- ↑ 1.0 1.1 Merck Index, 11th Edition, 4385
- ↑ 2.0 2.1 2.2 2.3 2.4 NIOSH Pocket Guide to Chemical Hazards. "#0303". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0303.html.
- ↑ 3.0 3.1 3.2 3.3 Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Food and Cosmetics Toxicology. Vol. 19, Pg. 347, 1981
- ↑ AMA Archives of Industrial Health. Vol. 14, Pg. 250, 1956
- ↑ "Glycidol". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/556525.html.
- ↑ Guenter Sienel, Robert Rieth, Kenneth T. Rowbottom "Epoxides" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a09_531
- ↑ Glycidol at chemicalland21.com
- ↑ "OSHA guidelines for glycidol". http://www.osha.gov/SLTC/healthguidelines/glycidol/recognition.html.
- ↑ "List of Classifications, Agents classified by the IARC Monographs, Volumes 1–124". IARC. July 7, 2019. https://monographs.iarc.fr/list-of-classifications.
- ↑ CDC - NIOSH Pocket Guide to Chemical Hazards
- ↑ Cheng, Wei-wei; Liu, Guo-qin; Wang, Li-qing; Liu, Zeng-she (2017). "Glycidyl Fatty Acid Esters in Refined Edible Oils: A Review on Formation, Occurrence, Analysis, and Elimination Methods" (in en). Comprehensive Reviews in Food Science and Food Safety 16 (2): 263–281. doi:10.1111/1541-4337.12251. ISSN 1541-4337. PMID 33371535.
 |

