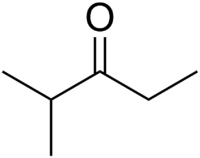
Chemistry:Ethyl isopropyl ketone
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methylpentan-3-one | |
| Other names
Ethyl isopropyl ketone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H12O | |
| Molar mass | 100.161 g·mol−1 |
| Density | 0.811 g/cm3[1] |
| Melting point | < 25 °C (77 °F; 298 K) |
| Boiling point | 113 °C (235 °F; 386 K)[1] |
| 15.5 mg/mL[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Ethyl isopropyl ketone (2-methyl-3-pentanone) is an aliphatic ketone with used as a reagent in organic chemistry and as a solvent.
Its fully fluorinated analog is known as Novec 1230 and is used in gaseous fire suppression.
References
- ↑ 1.0 1.1 "2-Methyl-3-pentanone". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/108707?lang=en®ion=US.
- ↑ "Ethyl isopropyl ketone (HMDB0005846)". Human Metabolome Database. http://www.hmdb.ca/metabolites/HMDB0005846.
 |

