Chemistry:Trisulfane
From HandWiki
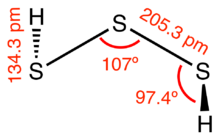
| |
| Names | |
|---|---|
| Systematic IUPAC name
Trisulfane[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| 3903006 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| 25473 | |
PubChem CID
|
|
| |
| |
| Properties | |
| H2S3 | |
| Molar mass | 98.20 g·mol−1 |
| Appearance | yellow liquid |
| Density | 1.495 g/cm3 (15 °C)[2] |
| Melting point | −53 °C (−63 °F; 220 K) |
| Boiling point | 170 °C (338 °F; 443 K) |
| low | |
| log P | 1.237 |
| Acidity (pKa) | 5.826 |
| Basicity (pKb) | 8.171 |
| Related compounds | |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Trisulfane is the inorganic compound with the formula H
2S
3. It is a pale yellow volatile liquid with a camphor-like odor. It decomposes readily to hydrogen sulfide (H
2S) and elemental sulfur. It is produced by distillation of the polysulfane oil obtained by acidification of polysulfide salts.[3]
References
- ↑ "trisulfane (CHEBI:50365)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute. 18 August 2008. Main. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=50365. Retrieved 27 September 2011.
- ↑ Feher, Franz; Baudler, Marianne. Chemistry of sulfur. III. The preparation and properties of hydrogen trisulfide. Zeitschrift für Anorganische Chemie, 1947. 254: 251-254. ISSN: 0372-7874.
- ↑ R. Steudel "Inorganic Polysulfanes H2Sn with n > 1" in Elemental Sulfur and Sulfur-Rich Compounds II (Topics in Current Chemistry) 2003, Volume 231, pp 99-125. doi:10.1007/b13182
 |

