Chemistry:Magnesium stearate
| |
| Names | |
|---|---|
| IUPAC name
Magnesium octadecanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
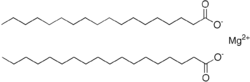
| Mg(C18H35O2)2 | |
| Molar mass | 591.27 g/mol |
| Appearance | light white powder |
| Odor | slight |
| Density | 1.026 g/cm3 |
| Melting point | 88.5 °C (191.3 °F; 361.6 K) |
| 0.003 g/100 mL (15 °C) 0.004 g/100 mL (25 °C) 0.008 g/100 mL (50 °C) | |
| Solubility | negligible in ether and alcohol slightly soluble in benzene |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 (fire diamond) | |
| Flash point | 250 °C (482 °F; 523 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 1000 mg/kg (oral, rat) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium stearate is the chemical compound with the formula Mg(C18H35O2)2. It is a soap, consisting of salt containing two equivalents of stearate (the anion of stearic acid) and one magnesium cation (Mg2+). Magnesium stearate is a white, water-insoluble powder. Its applications exploit its softness, insolubility in many solvents, and low toxicity. It is used as a release agent and as a component or lubricant in the production of pharmaceuticals and cosmetics.[1]
Manufacturing
Magnesium stearate is produced by the reaction of sodium stearate with magnesium salts or by treating magnesium oxide with stearic acid.[1][2]
Uses
Magnesium stearate is often used as an anti-adherent[3] in the manufacture of medical tablets, capsules and powders.[4] In this regard, the substance is also useful because it has lubricating properties, preventing ingredients from sticking to manufacturing equipment during the compression of chemical powders into solid tablets; magnesium stearate is the most commonly used lubricant for tablets.[5] However, it might cause lower wettability and slower disintegration of the tablets and slower and even lower dissolution of the drug.[6]
Magnesium stearate can also be used efficiently in dry coating processes.[7][8][9]
In the production of pressed candies, magnesium stearate serves as a release agent. It is also used to bind sugar in hard candies such as mints.[10]
Magnesium stearate is a common ingredient in baby formulas.[11]
In the EU and EFTA it is listed as food additive E470b.
Occurrence
Magnesium stearate is a major component of bathtub rings[citation needed]. When produced by soap and hard water, magnesium stearate and calcium stearate both form a white solid insoluble in water, and are collectively known as soap scum.
Safety
Magnesium stearate is generally considered safe for human consumption at levels below 2500 mg per kg of body weight per day[12] and is classified in the United States as generally recognized as safe (GRAS). In 1979, the FDA's Subcommittee on GRAS Substances (SCOGS) reported, "There is no evidence in the available information on ... magnesium stearate ... that demonstrates, or suggests reasonable grounds to suspect, a hazard to the public when they are used at levels that are now current and in the manner now practiced, or which might reasonably be expected in the future."[13]
References
- ↑ 1.0 1.1 Angelo Nora, Alfred Szczepanek, Gunther Koenen, "Metallic Soaps" in Ullmann's Encyclopedia of Industrial Chemistry 2005 Wiley-VCH, Weinheim. doi:10.1002/14356007.a16_361
- ↑ A. G. Dobson and H. H. Hatt (1953). "Stearone". Organic Syntheses 33: 84. doi:10.15227/orgsyn.033.0084.
- ↑ Ritter, Steve (2008). "What's That Stuff? Excipients: Inactive ingredients in medicines serve multiple functions in drug delivery". Chemical & Engineering News 86 (1): 25. doi:10.1021/cen-v086n001.p025. http://pubs.acs.org/cen/whatstuff/86/8601sci3.html.
- ↑ Sworbrick, James; Boylan, James C. (1990). Encyclopedia of pharmaceutical technology. p. 2274. ISBN 9780824728243.
- ↑ Weiner, Myra L.; Kotkoskie, Lois A. (1999). Excipient Toxicity and Safety. Taylor & Francis. p. 10. ISBN 9780824782108. https://archive.org/details/excipienttoxicit103wein/page/10.
- ↑ Demuth (2017). "Investigation of Deteriorated Dissolution of Amorphous Itraconazole: Description of Incompatibility with Magnesium Stearate and Possible Solutions". Molecular Pharmaceutics 14 (11): 3927–3934. doi:10.1021/acs.molpharmaceut.7b00629. PMID 28972782. https://www.researchgate.net/publication/320207928.
- ↑ Ouabbas Y, Dodds J., Galet L., Chamayou A. , Baron M. (2009). "Particle-particle coating in a cyclomix impact mixer". Powder Technol. 189 (2): 245–252. doi:10.1016/j.powtec.2008.04.031. https://hal.archives-ouvertes.fr/hal-01593335/file/particle-particle-coating.pdf.
- ↑ Thomas G., Ouabbas Y., Grosseau P., Baron M., Chamayou A., Galet L. (2009). "Modeling the main interaction forces between powder particles. Application to silica gel-magnesium stearate mixtures". Applied Surface Science 255 (17): 7500–7507. doi:10.1016/j.apsusc.2009.03.099. Bibcode: 2009ApSS..255.7500T.
- ↑ Sato A., Serris E., Grosseau P., Thomas G., Galet L., Chamayou A. , Baron M. (2013). "Experiment and simulation of dry particle coating". Chem. Eng. Science 86: 164–172. doi:10.1016/j.ces.2012.07.037. Bibcode: 2013ChEnS..86..164S. https://hal.archives-ouvertes.fr/hal-00616515/file/AS-Lausanne-Orig.pdf.
- ↑ https://www.ctahr.hawaii.edu/oc/freepubs/pdf/FST-9.pdf [bare URL PDF]
- ↑ Erich Lück and Gert-Wolfhard von Rymon Lipinski (2002). "Foods, 3. Food Additives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_561. ISBN 978-3527306732.
- ↑ Søndergaarda, D.; Meyera, O.; Würtzena, G. (1980). "Magnesium stearate given peroprally to rats. A short term study". Toxicology 17 (1): 51–55. doi:10.1016/0300-483X(80)90026-8. PMID 7434368.
- ↑ FDA's SCOGS Database; Report No. 60; ID Code: 557-04-0; Year: 1979
 |


