Chemistry:Cyclohexylmethanol
From HandWiki
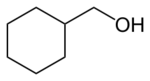
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexylmethanol | |
| Other names
Cyclohexanemethanol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H14O | |
| Molar mass | 114,19 g·mol−1 |
| Appearance | colorless liquid with a smell of alcohol[1] |
| Density | 0,9339 g·cm−3[2] |
| Melting point | 19 °C (66 °F)[1] |
| Boiling point | 187–188 °C (369–370 °F)[3] |
| small in water[4] | |
| Hazards | |
| Flash point | 71 °C (160 °F).[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Cyclohexylmethanol is an organic compound with the formula C
6H
11–CH
2–OH. It is a cyclohexane ring functionalized with an alcohol, specifically a hydroxymethyl group. The compound is a colorless liquid, although commercial samples can appear yellow.
Production
Cyclohexylmethanol can be produced in two step starting with the hydroformylation of cyclohexene. This process also give cyclohexane, resulting from hydrogenation. The resulting cyclohexanecarboxaldehyde is then hydrogenated to give the alcohol.[5][6]
References
- ↑ 1.0 1.1 1.2 Record of Cyclohexylmethanol in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 4 October 2014..
- ↑ Prey, Vinzenz; Bartsch, Jürgen (1968). "Dipolmessungen an Pyranose- und Furanose-Modellsubstanzen" (in de). Justus Liebigs Annalen der Chemie (Wiley-VCH) 712 (1): 201–207. doi:10.1002/jlac.19687120124.
- ↑ Rickborn, Bruce; Wood, Stanley E. (1971). "Cleavage of cyclopropanes by diborane". Journal of the American Chemical Society (American Chemical Society) 93 (16): 3940–3946. doi:10.1021/ja00745a021.
- ↑ Ruelle, Paul; Kesselring, Ulrich W. (February 1997). "The Hydrophobic Propensity of Water toward Amphiprotic Solutes: Predicton and Molecular Origin of the Aqueous Solubility of Aliphatic Alcohols". Journal of Pharmaceutical Sciences (American Pharmacists Association, Elsevier) 86 (2): 179–186. doi:10.1021/js9603109. ISSN 0022-3549.
- ↑ "Method for Producing Alcohol by Using Carbon Dioxide as Raw MaterialL" EP patent 2000453, published 2008-12-10.
- ↑ Feng, Jinhai; Garland, Marc (1999). "The Unmodified Homogeneous Rhodium-Catalyzed Hydroformylation of Cyclohexene and the Search for Monometallic Catalytic Binuclear Elimination". Organometallics (American Chemical Society) 18 (8): 1542–1546. doi:10.1021/om980531k. ISSN 1520-6041.
 |

