Chemistry:TosMIC
From HandWiki
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
1-(Isocyanomethanesulfonyl)-4-methylbenzene | |
| Other names
Toluenesulfonylmethyl isocyanide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C9H9NO2S | |
| Molar mass | 195.24 g·mol−1 |
| Melting point | 109 to 113 °C (228 to 235 °F; 382 to 386 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H301, H311, H331 | |
| P261, P264, P270, P271, P280, P301+310, P302+352, P304+340, P311, P312, P321, P322, P330, P361, P363, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
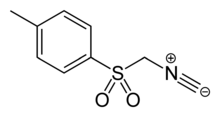
TosMIC (toluenesulfonylmethyl isocyanide) is an organic compound with the formula CH3C6H4SO2CH2NC. The molecule contains both sulfonyl and isocyanide groups. It is a colourless solid that, unlike many isocyanides, is odorless. It is prepared by dehydration of the related formamide derivative. It is used in the Van Leusen reaction which is used to convert ketones to nitriles or in the preparation of oxazoles[2] and imidazoles.[3] The versatility of TosMIC in organic synthesis has been documented.[4] It is a fairly strong carbon acid, with an estimated pKa of 14 (compared to 29 for methyl tolyl sulfone), the isocyano group acting as an electron acceptor of strength comparable to an ester group.[5]
Further reading
References
- ↑ p-Toluenesulfonylmethyl isocyanide at Sigma-Aldrich
- ↑ Keeri, Abdul Raheem; Gualandi, Andrea; Mazzanti, Andrea; Lewinski, Janusz; Cozzi, Pier Giorgio (2015-12-21). "Me2Zn-Mediated Catalytic Enantio- and Diastereoselective Addition of TosMIC to Ketones" (in en). Chemistry – A European Journal 21 (52): 18949–18952. doi:10.1002/chem.201504362. ISSN 1521-3765. PMID 26549317.
- ↑ Hoogenboom, B. E.; Oldenziel, O. H.; van Leusen, A. M. (1977). "p-TOLYLSULFONYLMETHYL ISOCYANIDE". Organic Syntheses 57: 102. http://www.orgsyn.org/demo.aspx?prep=CV6P0987.; Collective Volume, 6, pp. 987
- ↑ "Toluenesulphonylmethyl isocyanide (TOSMIC) and the van Leusen MCR". https://www.organic-chemistry.org/Highlights/2005/05May.shtm.
- ↑ van Leusen, Albert M.; van Leusen, Daan; Czakó, Barbara (2008-09-15) (in en), p-Tolylsulfonylmethyl Isocyanide, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rt150.pub2, ISBN 978-0471936237
- ↑ Leusen, Daan Van; Leusen, Albert M. Van (2001). "Synthetic Uses of Tosylmethyl Isocyanide (TosMIC)". Organic Reactions. pp. 417–666. doi:10.1002/0471264180.or057.03. ISBN 0471264180.
 |

