Chemistry:Sinigrin
| |
| Names | |
|---|---|
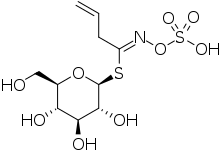
| IUPAC name
(Z)-N-[1-(β-D-glucopyranosylsulfanyl)but-3-en-1-ylidene]hydroxylamine-O-sulfonic acid
| |
| Systematic IUPAC name
(Z)-N-(1-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}but-3-en-1-ylidene)hydroxylamine-O-sulfonic acid | |
| Other names
Allyl glucosinolate; 2-Propenyl glucosinolate; (1Z)-N-(Sulfooxy)but-3-enimidoyl 1-thio-β-D-glucopyranoside
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C10H17NO9S2 | |
| Molar mass | 359.36 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sinigrin or allyl glucosinolate is a glucosinolate that belongs to the family of glucosides found in some plants of the family Brassicaceae such as Brussels sprouts, broccoli, and the seeds of black mustard (Brassica nigra). Whenever sinigrin-containing plant tissue is crushed or otherwise damaged, the enzyme myrosinase degrades sinigrin to a mustard oil (allyl isothiocyanate), which is responsible for the pungent taste of mustard and horseradish.[1] Seeds of white mustard, Sinapis alba, give a less pungent mustard because this species contains a different glucosinolate, sinalbin.
Occurrence
The compound was first reported in 1839,[2] after its isolation from black mustard Brassica nigra, also known as Sinapis nigra, after which it was named.[3]:Section 2 Sinigrin is now known to occur widely in other brassica families including Brassicaceae and Capparaceae.[4]
Structure
The chemical structure of sinigrin had been established by 1930. This showed that it is a glucose derivative with β-D-glucopyranose configuration. It was unclear whether the C=N bond was in the Z (or syn) form, with sulfur and oxygen substituents on the same side of the double bond, or the alternative E form in which they are on opposite sides. The matter was settled by X-ray crystallography of its potassium salt in 1963.[5][6] It is now known that all natural glucosinolates are of Z form.[3]
Synthesis
Biosynthesis
Sinigrin is biosynthesised from the amino acid methionine in a multi-step pathway.[3]
Laboratory synthesis
The first laboratory syntheses of sinigrin was published in 1965.[2] Later work provided a more efficient route.[7][3]:Section 3
Function
The natural role of glucosinolates are as plant defense compounds. The enzyme myrosinase removes the glucose group in sinigrin to give an intermediate which spontaneously rearranges to allyl isothiocyanate, the compound responsible for the pungent taste of Dijon mustard. This is a reactive material which is toxic to many insect predators and its production is triggered when the plant is damaged.[8] This effect has been called the mustard oil bomb.[9] Singrin is also known to be allelopathic.[10] At concentrations typically found in foods, the glucosinolates are not toxic to humans and can be useful flavor components.[11]
Sinigrin is unusual among the glucosinolates because it is also known to be the natural precursor for other volatile compounds including epithionitrile, allyl cyanide and allyl thiocyanate.[3]:Fig. 22
See also
References
- ↑ Richard, H.. "Arômes alimentaires" (in fr). http://www.cnrs.fr/chimie/communication/images/images-chimietous/minidossiers/cours%20aromes_hubert%20richard.pdf.
- ↑ Jump up to: 2.0 2.1 Benn, M. H.; Ettlinger, M. G. (1965). "The synthesis of sinigrin". Chemical Communications (19): 445. doi:10.1039/C19650000445.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 Blažević, Ivica; Montaut, Sabine; Burčul, Franko; Olsen, Carl Erik; Burow, Meike; Rollin, Patrick; Agerbirk, Niels (2020). "Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants". Phytochemistry 169: 112100. doi:10.1016/j.phytochem.2019.112100. PMID 31771793. Bibcode: 2020PChem.169k2100B.
- ↑ Fahey, Jed W.; Zalcmann, Amy T.; Talalay, Paul (2001). "The chemical diversity and distribution of glucosinolates and isothiocyanates among plants". Phytochemistry 56 (1): 5–51. doi:10.1016/S0031-9422(00)00316-2. PMID 11198818. Bibcode: 2001PChem..56....5F.
- ↑ Waser, Jürg; Watson, William H. (1963). "Crystal Structure of Sinigrin". Nature 198 (4887): 1297–1298. doi:10.1038/1981297b0. Bibcode: 1963Natur.198.1297W.
- ↑ Marsh, R. E.; Waser, J. (1970). "Refinement of the crystal structure of sinigrin". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry 26 (7): 1030–1037. doi:10.1107/S0567740870003539. Bibcode: 1970AcCrB..26.1030M. https://authors.library.caltech.edu/70930/.
- ↑ Abramski, Wojciech; Chmielewski, Marek (1996). "Practical Synthesis of Sinigrin". Journal of Carbohydrate Chemistry 15: 109–113. doi:10.1080/07328309608005429.
- ↑ Morant, Anne Vinther; Jørgensen, Kirsten; Jørgensen, Charlotte; Paquette, Suzanne Michelle; Sánchez-Pérez, Raquel; Møller, Birger Lindberg; Bak, Søren (2008). "β-Glucosidases as detonators of plant chemical defense". Phytochemistry 69 (9): 1795–1813. doi:10.1016/j.phytochem.2008.03.006. PMID 18472115. Bibcode: 2008PChem..69.1795M.
- ↑ Matile, Ph. (1980). ""Die Senfolbombe": Zur Kompartimentierung des Myrosinasesystems" (in de). Biochemie und Physiologie der Pflanzen 175 (8–9): 722–731. doi:10.1016/S0015-3796(80)80059-X.
- ↑ Lankau, Richard (2008). "A Chemical Trait Creates a Genetic Trade-Off Between Intra- and Interspecific Competitive Ability". Ecology 89 (5): 1181–1187. doi:10.1890/07-1541.1. PMID 18543611.
- ↑ Fenwick, G. Roger; Heaney, Robert K.; Mullin, W. John; Vanetten, Cecil H. (1983). "Glucosinolates and their breakdown products in food and food plants". C R C Critical Reviews in Food Science and Nutrition 18 (2): 123–201. doi:10.1080/10408398209527361. PMID 6337782.
 |


