Chemistry:Tert-Butyl hypochlorite
From HandWiki
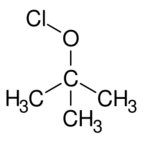
| |
| Names | |
|---|---|
| Preferred IUPAC name
tert-Butyl hypochlorite | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| UN number | 3255 |
| |
| |
| Properties | |
| C4H9ClO | |
| Molar mass | 108.57 g·mol−1 |
| Appearance | Yellow liquid |
| Density | 0.9583 g/cm3 |
| Boiling point | 79.6 °C (175.3 °F; 352.8 K) explosive |
| Sparingly | |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
| H225, H250, H251, H271, H314, H334 | |
| P210, P220, P221, P222, P233, P235+410, P240, P241, P242, P243, P260, P261, P264, P280, P283, P285, P301+330+331, P302+334, P303+361+353, P304+340, P304+341, P305+351+338, P306+360, P310, P321 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
tert-Butyl hypochlorite is the organic compound with the formula (CH3)3COCl. A yellow liquid, it is a rare example of an organic hypochlorite, i.e. a compound with an O-Cl bond. It is a reactive material that is useful for chlorinations. It can be viewed as a lipophilic version of sodium hypochlorite (bleach).[1]
Synthesis and reactions
It is produced by chlorination of tert-butyl alcohol in the presence of base:[2][3]
- (CH
3)
3COH + Cl
2 + NaOH → (CH
3)
3COCl + NaCl + H
2O
tert-Butyl hypochlorite is useful in the preparation of organic chloramines:[4]
- R2NH + t-BuOCl → R2NCl + t-BuOH
References
- ↑ Simpkins, Nigel S.; Cha, Jin K. (2006). "t-Butyl Hypochlorite". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rb388.pub2. ISBN 0471936235.
- ↑ Teeter, H. M.; Bell, E. W. (1952). "tert-Butyl Hypochlorite". Org. Synth. 32: 20. doi:10.15227/orgsyn.032.0020.
- ↑ Mintz, H. M.; Walling, C. (1969). "t-Butyl Hypochlorite". Org. Synth. 49: 9. doi:10.15227/orgsyn.049.0009.
- ↑ Herranz, Eugenio; Sharpless, K. Barry (1983). "Osmium-catalyzed Vicinal Oxyamination of Olefins by N-chloro-N-Argentocarbamates: Ethyl Threo-[1-(2-hydroxy-1,2-diphenylethyl)]carbamate". Org. Synth. 61: 93. doi:10.15227/orgsyn.061.0093.
 |

