Chemistry:Nangibotide
 | |
| Clinical data | |
|---|---|
| Routes of administration | Intravenous; intraperitoneal |
| Physiology data | |
| Receptors | TREM-1 |
| Metabolism | Enzymatic in bloodstream |
| Pharmacokinetic data | |
| Metabolism | Enzymatic in bloodstream |
| Elimination half-life | 3 minutes |
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
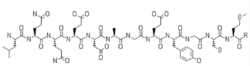
| Formula | C54H82N14O22S2 |
| Molar mass | 1343.439 |
| 3D model (JSmol) | |
| |
| |
Nangibotide is an inhibitor of TREM-1, a receptor found on certain white blood cells. Activation of TREM-1 stimulates inflammation. Nangibotide is therefore being investigated as a treatment for the overwhelming inflammation typically seen in severe sepsis.
Chemistry
Nangibotide is a 12-amino-acid polypeptide derived from TLT-1.[1]
Mode of action
TREM-1 is a receptor found on neutrophils, macrophages and monocytes, key elements of the immune system. Activation of TREM-1 results in expression of NF-κB, which promotes systemic inflammation. Nangibotide inhibits TREM-1, thereby preventing the inflammatory activation. Absence of TREM-1 results in vastly reduced inflammation without impairing the ability to fight infection.[2]
Animal models
LR17, a mouse equivalent of nangibotide, improves survival in mouse models of severe sepsis.[3] In a pig model of sepsis, LR12 - another animal equivalent of nangibotide - resulted in significantly improved haemodynamics and less organ failure.[4] In monkeys, LR12 also reduced the inflammatory and hypotensive effects of sepsis.[5]
Human studies
Nangibotide has demonstrated safety in Phase 1 (healthy volunteers)[6] and Phase 2 (sick patients with septic shock)[7] studies. The ASTONISH trial will examine clinical efficacy in 450 patients with septic shock.[8]
References
- ↑ "A first-in-man safety and pharmacokinetics study of nangibotide, a new modulator of innate immune response through TREM-1 receptor inhibition.". British Journal of Clinical Pharmacology 84 (10): 2270–2279. 2018. doi:10.1111/bcp.13668. PMID 29885068.
- ↑ "TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance.". PLOS Pathog. 10 (1): e1003900. 2014. doi:10.1371/journal.ppat.1003900. PMID 24453980.
- ↑ "Soluble TREM-like transcript-1 regulates leukocyte activation and controls microbial sepsis.". Journal of Immunology 188 (11): 5585–5592. 1 June 2012. doi:10.4049/jimmunol.1102674. PMID 22551551.
- ↑ "Effects of a TREM-like transcript 1-derived peptide during hypodynamic septic shock in pigs.". Shock 39 (2): 176–182. Feb 2013. doi:10.1097/SHK.0b013e31827bcdfb. PMID 23324887. https://pubmed.ncbi.nlm.nih.gov/23324887/.
- ↑ "Attenuation of responses to endotoxin by the triggering receptor expressed on myeloid cells-1 inhibitor LR12 in nonhuman primate.". Anesthesiology 120 (4): 935–942. April 2014. doi:10.1097/ALN.0000000000000078. PMID 24270127. https://pubmed.ncbi.nlm.nih.gov/24270127/.
- ↑ "A first-in-man safety and pharmacokinetics study of nangibotide, a new modulator of innate immune response through TREM-1 receptor inhibition.". Br J Clin Pharmacol 84 (10): 2270–2279. 2018. doi:10.1111/bcp.13668. PMID 29885068.
- ↑ "Nangibotide in patients with septic shock: a Phase 2a randomized controlled clinical trial.". Intensive Care Medicine 46 (7): 1425–1437. July 2020. doi:10.1007/s00134-020-06109-z. PMID 32468087. https://pubmed.ncbi.nlm.nih.gov/32468087/.
- ↑ "Efficacy, Safety and Tolerability of Nangibotide in Patients With Septic Shock (ASTONISH)". US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT04055909.
 |

