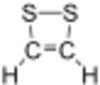
Chemistry:Dithiete
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,2-Dithiete | |
| Systematic IUPAC name
1,2-Dithiacyclobut-3-ene | |
| Other names
Dithiete
Dithiacyclobutene 1,2-Dithia[4]annulene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C2H2S2 | |
| Molar mass | 90.16 g·mol−1 |
| Related compounds | |
Related thietes
|
Thiete |
Related compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dithiete is an unsaturated heterocyclic compound that contains two adjacent sulfur atoms and two sp2-hybridized carbon centers. Derivatives are known collectively as dithietes or 1,2-dithietes. With 6 π electrons, 1,2-dithietes are examples of aromatic organosulfur compounds. A few 1,2-dithietes have been isolated.[1][2][3] 3,4-Bis(trifluoromethyl)-1,2-dithiete is a particularly stable example.
Unsubstituted 1,2-dithiete has been generated in thermolytic reactions and was characterized by microwave spectroscopy, ultraviolet photoelectron spectroscopy and infrared spectroscopy in a low temperature matrix. The open ring isomer, dithioglyoxal, HC(S)C(S)H, is less stable than the 1,2-dithiete.[4]
The dithione can be prepared (as trans-dithioglyoxal) by low temperature photolysis of 1,3-dithiol-2-one.[5] Quantum chemical calculations reproduce the observed greater stability of 1,2-dithiete only if large basis-sets with polarization functions are used.[6]
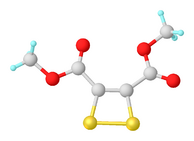 Structure of the dithiete S2C2(CO2Me)2. Selected distances and angles: rC=C = 1.362, rC-S = 1.738, rS-S = 2.072 Å, <S-S-C = 78.3°, <S-C-S = 102°.[7]
Structure of the dithiete S2C2(CO2Me)2. Selected distances and angles: rC=C = 1.362, rC-S = 1.738, rS-S = 2.072 Å, <S-S-C = 78.3°, <S-C-S = 102°.[7]
See also
- Dithietane - the corresponding saturated ring
- Thiete - an analogue with only one sulfur atom
Additional reading
- Diehl, F.; Meyer, H.; Schweig, A.; Hess, B. A.; Fabian, J. (September 1989). "1,2-Dithiete is more stable than 1,2-dithioglyoxal as evidenced by a combined experimental and theoretical IR spectroscopic approach". J. Am. Chem. Soc. 111 (19): 7651–7653. doi:10.1021/ja00201a076.
- Vijay, D; Priyakumar, UD; Sastry, GN (2004). "Basis set and method dependence of the relative energies of C2S2H2 isomers". Chemical Physics Letters 383 (1–2): 192–197. doi:10.1016/j.cplett.2003.11.021. Bibcode: 2004CPL...383..192V.
- Jonas, V; Frenking, G (1991). "On the crucial importance of polarization functions for the calculation of molecules with third-row elements: the conformations of chlorocarbonyl isocyanate ClC(O)NCO and the equilibrium of 1,2-dithioglyoxal with its cyclic isomer 1,2-dithiete". Chemical Physics Letters 177 (2): 175–183. doi:10.1016/0009-2614(91)90064-G. Bibcode: 1991CPL...177..175J.
- Gonzalez, L; Mo, O; Yanez, M (13 December 1996). "High-level ab initio calculations on the 1,2-dithioglyoxal/1,2-dithiete isomerism". Chemical Physics Letters 263 (3): 407–413(7). doi:10.1016/S0009-2614(96)01240-7. Bibcode: 1996CPL...263..407G.
References
- ↑ Drabowicz, J; Lewkowski, J; Kudelska, W; Zając, A (2008). "Four-membered Rings with Two Sulfur Atoms". Comprehensive Heterocyclic Chemistry III. 2. pp. 811–852. doi:10.1016/B978-008044992-0.00218-2. ISBN 978-0-08-044992-0.
- ↑ Zoller, U (1996). "Four-membered Rings with Two Sulfur Atoms". Comprehensive Heterocyclic Chemistry II. 1B. pp. 1113–1138. doi:10.1016/B978-008096518-5.00035-6. ISBN 978-0-08-096518-5.
- ↑ Donahue, JP; Holm, RH (1998). "3,4-Bis(1-adamantyl)-1,2-dithiete: the First Structurally Characterized Dithiete Unsupported by a Ring or Benzenoid Frame". Acta Crystallographica C54 (8): 1175–1178. doi:10.1107/S0108270198002935. PMID 9760719.
- ↑ Reinhard Schulz; Armin Schweig; Klaus Hartke; Joachim Koester (1983). "Theory and application of photoelectron spectroscopy. 100. Variable-temperature photoelectron spectral study of 1,3-dithiol-2-one and 4,5-disubstituted 1,3-dithiol-2-ones. Thermal generation of 1,2-dithiete, 3,4-disubstituted 1,2-dithietes, and dialkyl tetrathiooxalates". Journal of the American Chemical Society 105 (14): 4519–4528. doi:10.1021/ja00352a004.
- ↑ Mucha, M; Pagacza, M; Mielke, Z (6 June 2008). "Infrared detection of dithioglyoxal from photolysis of 1,3-dithiol-2-one in solid argon and nitrogen". Chemical Physics Letters 458 (1–3): 39–43. doi:10.1016/j.cplett.2008.04.088. Bibcode: 2008CPL...458...39M.
- ↑ Frolov, YV; Vashchenko, AV; Mal'kina, AG; Trofimov, BA (2009). "Ab initio quantum-chemical calculations of the energies and structures of 1,2-acetylenedithiol isomers". Journal of Structural Chemistry 50 (2): 195–200. doi:10.1007/s10947-009-0029-8.
- ↑ T. Shimizu; H. Murakami; Y. Kobayashi; K. Iwata; N. Kamigata (1998). "Synthesis, Structure, and Ring Conversion of 1,2-Dithiete and Related Compounds". J. Org. Chem. 63 (23): 8192–8199. doi:10.1021/jo9806714.
 |

