Chemistry:Chinese lantern structure
From HandWiki
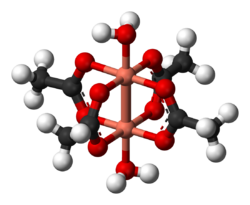
In chemistry, the Chinese lantern structure is a coordination complex where two metal atoms are bridged by four bidentate ligands. This structure type is also known as a paddlewheel complex. Examples include chromium(II) acetate, molybdenum(II) acetate, and rhodium(II) acetate, copper(II) acetate dihydrate. The name is derived from the resemblance between the structure and a Chinese paper lantern. Often additional ligands are bound to the metal centers along the M---M vector. The degree of metal-metal bonding varies according to the d-electron configuration.[1]
Complexes with Chinese lantern structure
- Copper benzoate
- Copper acetate
- Chromium(II) acetate
- Molybdenum(II) acetate
- Diruthenium tetraacetate chloride
- Rhodium acetate
References
- ↑ Cotton, F. A.; Walton, R. A. (1993). Multiple Bonds Between Metal Atoms. Oxford: Oxford University Press. ISBN 0-19-855649-7. https://archive.org/details/multiplebondsbet0000cott.
Further reading
- Talismanova, M. O.; Sidorov, A. A.; Aleksandrov, G. G.; Oprunenko, Yu. F.; Eremenko, I. L.; Moiseev, I. I. (2004). "New dinuclear palladium complex with a Chinese-lantern structure". Russian Chemical Bulletin 53 (7): 1507. doi:10.1023/B:RUCB.0000046248.72380.79.
 |